INTRODUCTION
Artificial insemination (AI) is a widely used technique for animal reproduction in the livestock industry. Researchers are focusing on developing innovative methods for liquid preservation to enhance sperm quality [1]. Studies have shown that motility, viability, acrosome integrity, chromatin stability, and levels of reactive oxygen species (ROS) are the primary parameters essential for maintaining sperm quality and fertilization ability [2]. During storage, metabolic pathways in the spermatozoa result in the production of byproducts including ROS, superoxide anion, and hydrogen peroxide [3]. The excessive generation of byproducts in an in vitro environment decreases sperm quality and affects fertilization [4]. Therefore, it is crucial to include relevant substances in semen extenders to mitigate the negative impact of these byproducts [5]. Importantly, these substances should be capable of maintaining energy production and redox potential to ensure sperm quality [6].
The addition of antioxidants to semen extenders is a common approach used to maintain the boar sperm quality during prolonged storage [7, 8]. Antioxidants, including fish oil, vitamin E, organic selenium, and ginseng extract, have been proven to enhance antioxidant activity and maintain the quality of boar spermatozoa during storage [9–13]. Pyruvate is a natural substance produced as the final product of glycolysis. However, it is unstable in an aqueous solution. To resolve this issue, ethyl pyruvate (EP) was synthesized as a stable derivative of pyruvate [14, 15]. Research studies have shown that EP provides biological and pharmacological benefits, including antioxidant and anti-inflammatory effects, in both in vivo and in vitro research models [14, 16]. EP is associated with energy metabolism in cells, during which ROS are produced. Overproduction of ROS can damage cellular components, like DNA. EP has the ability to remove or neutralize the excess ROS [16–18]. In one study, EP was administered to mice with phenylhydrazine (PHZ)-induced hemolytic anemia and the sperm quality parameters were examined in comparison to the PHZ-only treated group [19]. The EP-treated group showed higher sperm motility, viability, and improved sperm morphology compared to the PHZ-induced hemolytic anemia group. These effects were attributed to the free radical scavenging ability of EP [19]. Similarly, administration of EP to cyclophosphamide-induced oxidative stress mice significantly increased sperm count, motility, viability, and normal morphology, and reduced DNA damage compared with the cyclophosphamide-only treated group [11].
Research studies have investigated the effect of EP on the sperm quality parameters of animals in vivo. However, no studies have been conducted to assess its effects on boar spermatozoa in vitro. Therefore, there is a research gap with respect to the effect of EP on boar spermatozoa during sperm preservation. The current study investigated the effect of EP on the quality parameters of boar spermatozoa during storage.
MATERIALS AND METHODS
Freshly collected boar semen samples from adult Duroc boars with sperm motility exceeding 80% were purchased from the local AI center. Boar sperm was washed and extended in Beltsville thawing solution (BTS), supplemented with different concentrations of EP (0.1, 0.25, 0.5, 0.75, 1 mM), or placed in a control group (without EP). EP concentrations in the range of 0.1 to 1 mM were selected based on preliminary experiments using boar sperm to evaluate their effects on sperm quality during storage. The samples were subjected to storage at 17°C for 5 days.
Sperm motility parameters were evaluated using a computer-assisted sperm analyzer (CASA, Sperm Class Analyzer, Microptic S.L., Barcelona, Spain). Before measuring the sperm motility, the Leja chamber slide was pre-warmed at 37°C. Subsequently, a 2 µL sample was placed into chamber slides (Leja Products B.V., Nieuw-Vennep, The Netherlands) and ten optical fields were analyzed, encompassing a total of not less than 500 spermatozoa. The CASA system assessed the following as motion kinetic parameters: progressive motility, linearity index, straightness index, curvilinear velocity, straight-line velocity, oscillation index, and average path velocity.
Spermatozoa were rinsed twice in phosphate-buffered saline (PBS) containing 0.1% (w/v) polyvinyl alcohol (PBS-PVA). Viability was determined by employing the sperm viability kit (LIVE/DEAD®, Molecular Probes, Eugene, OR, USA). First, spermatozoa were centrifuged at room temperature (RT) for 5 min, and then the supernatant was removed. Following that, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer was added to the sperm pellet and incubated with the SYBR14 stain (100 nM) for 5 min. Propidium iodide (PI, 10 μM) was added to the sample, and it was incubated for 5 min. The stained samples were mounted with a mounting medium and examined using a fluorescence microscope (Nikon, Tokyo, Japan), and images were captured (DS-Fi2, Nikon). Live sperm cells appeared green, while dead cells appeared red.
Spermatozoa were centrifuged twice with PBS at RT for 5 min each. After centrifugation, the spermatozoa were placed onto the slides. For cell fixation, the slides were incubated in 95% ethanol at 4°C for 30 min, after which the slides were allowed to air dry completely. For acrosome staining, fixed spermatozoa were incubated with fluorescein isothiocyanate-Pisum sativum agglutinin (FITC-PSA) at RT for 8–10 min. After incubation, the excess staining solution was removed, and the slides were subjected to three washes with PBS [20]. The samples were subsequently monitored under a fluorescence microscope coupled with an imaging system (Nikon Eclipse Ci microscope, Nikon). Spermatozoa exhibiting green fluorescence localized to the acrosome region were defined as intact acrosomes, while those lacking/absent green fluorescence were classified as acrosome-reacted or damaged.
Spermatozoa were centrifuged twice with PBS at RT for 5 min each. After centrifugation, spermatozoa were placed onto the slides. To fix the cells, the slides were incubated with Carnoy’s solution (methanol: glacial acetic acid = 3:1) for 15 min at 4°C and then air dried. The fixed slides were then incubated in a tampon solution (80 mM citric acid, 15 mM sodium hydrogen phosphate [Na2HPO4], pH 2.5) at 75°C for 5 min. Once cooled to RT, the slides were incubated with acridine orange solution (0.2 mg/mL) for 5 min at RT. The excess stain was gently tapped off, and the slides were subjected to two successive washes with distilled water [21]. The samples were then explored under a fluorescence microscope equipped with an imaging system (Nikon Eclipse Ci microscope, Nikon). Sperm emitting green fluorescence were classified as having normal chromatin integrity, while those showing yellow to red fluorescence were considered to have denatured DNA.
The spermatozoa were centrifuged twice with 0.1 PBS-PVA at RT for 5 min. The sperm pellet was then resuspended with 0.1 PBS-PVA solution and incubated with 1 µM 5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, Invitrogen, Eugene, OR, USA) at 37°C for 10 min. Following incubation, the spermatozoa were subjected to two washes with PBS and mounted on the slides [22]. ROS production was then examined under a fluorescence microscope (Nikon Eclipse Ci microscope, Nikon).
Statistical analyses were performed using one-way ANOVA via the GraphPad PRISM® software (San Diego, CA, USA). Our study utilized a completely randomized design, and significant differences between groups were assessed using Tukey’s multiple comparison test and the t-test. Data are represented as mean ± S.E.M. and statistical significance was set at *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
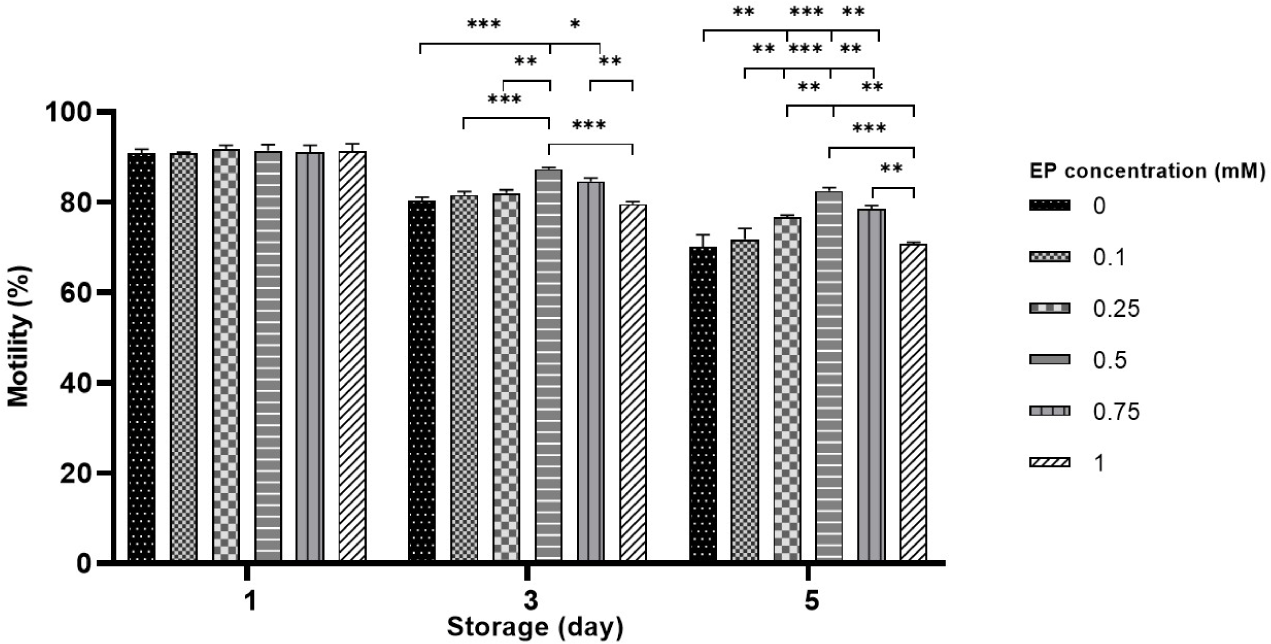
Sperm motility of boar spermatozoa was measured over 5 days in liquid storage without EP (control) and with EP (0.1–1 mM). On day 1 of storage, no statistically significant differences in motility were detected among the EP groups compared to the control. However, on day 3, a notable increase in motility was observed in the 0.5–0.75 mM EP groups compared to the control and 1 mM EP groups (79.6%–80.4% in the control vs. 84.5%–87.3% in 0.5–0.75 mM EP, p<0.05 and p<0.001; Fig. 1). On day 5, the sperm stored with 0.25–0.75 mM EP also showed significantly higher motility compared to the control and 1 mM EP group (70.7%–70% vs. 76.7%–82.5%, p<0.01 and p<0.001; Fig. 1). Notably, the highest motility was detected in sperm stored with 0.5 mM EP on both days 3 and 5. Furthermore, the EP-exposed groups exhibited marked improvements in motion kinetic parameters, including progressive motility, linearity index, straightness index, curvilinear velocity, straight-line velocity, oscillation index, and average path velocity compared to the control group during storage (p<0.05, p<0.01 and p<0.001; Table 1).
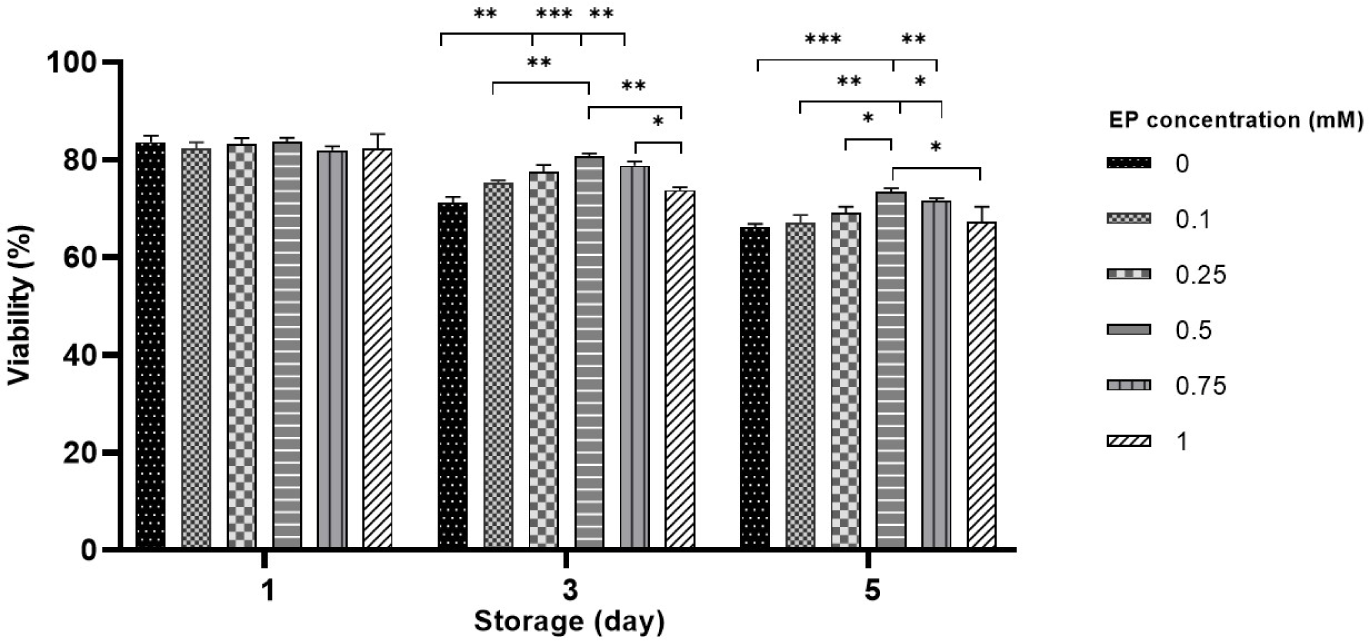
Sperm viability was evaluated throughout the liquid storage, and no pronounced differences were detected between the EP groups and control on day 1 (Fig. 2). After 3 days of storage, viability was notably higher in sperm stored with 0.25-0.75 mM EP compared to both control and the 1 mM EP group (71.1%–73.8% in control vs. 77.5%–80.8% in 0.25–0.75 mM EP, p<0.01 and p<0.001; Fig. 2). Compared to the control, 0.5–0.75 mM EP had a significantly higher effect on viability on day 5 (66.5% in control vs. 71.6%–73.5% in 0.5–0.75 mM EP, p<0.01 and p<0.001; Fig. 2). Consistent with the motility results, the 0.5 mM EP group showed the highest viability among all EP groups in both days 3 and 5. However, storage with 0.1 and 1 mM did not significantly improve viability compared with the control on either day 3 or 5 of liquid storage.
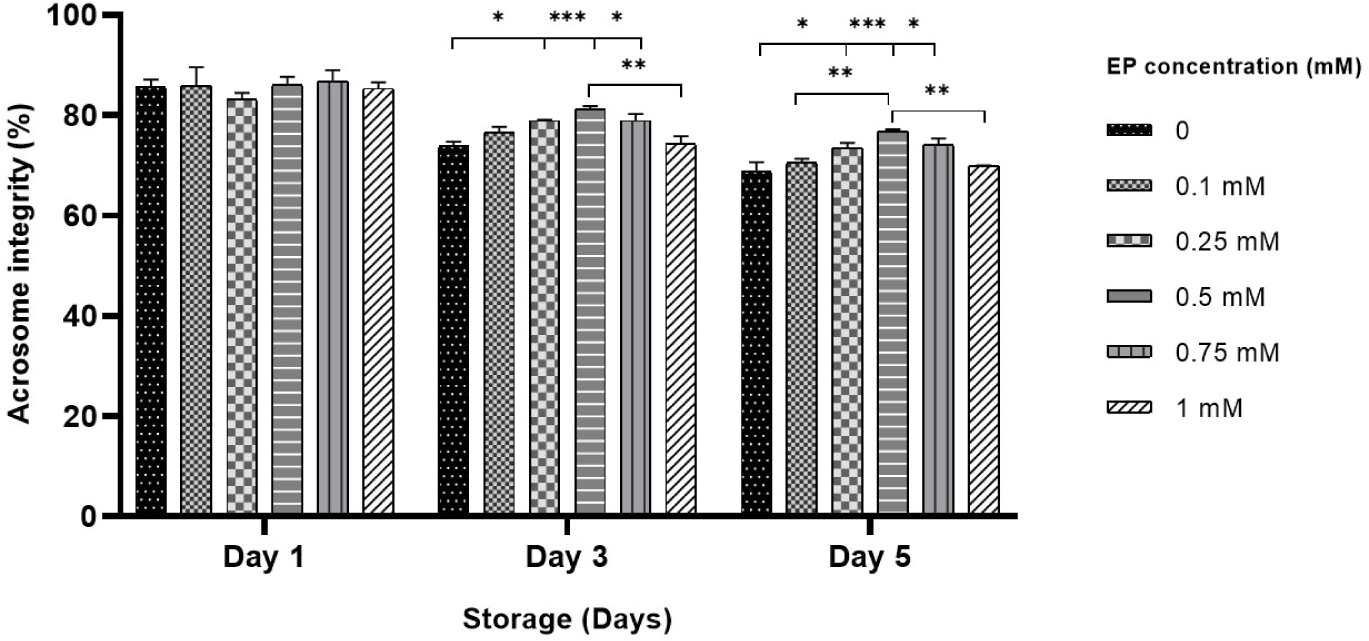
On day 1, there was no notable difference in the percentages of sperm with intact acrosome among the treatment groups (Fig. 3). By day 3, sperm stored with 0.25–0.75 mM EP exhibited a significant increase in the percentage of intact acrosome relative to the control group (73.8% in control vs. 78.9%–81.3% in 0.25–0.75 mM EP, p<0.05 and p<0.001; Fig. 3). Likewise, on day 5, supplementation with 0.25–0.75 mM EP in the BTS solution significantly improved the percentage of intact acrosome (68.8% in control vs. 73.5%–76.8% in 0.25–0.75 mM EP, p<0.05 and p<0.001; Fig. 3).
Chromatin stability was assessed over 5 days of storage. Consistent with the previous results, no differences were detected in the proportion of normal chromatin among the group on day 1 (Fig. 4). However, on day 3, sperm stored with 0.25–0.75 mM EP exhibited a significantly higher proportion of normal chromatin (71.1% in control vs 77.9%–80.6% in 0.25–0.75 mM EP, p<0.05, p<0.01 and p<0.001; Fig. 4). Sperm stored with 0.25–0.75 mM EP showed a statistically higher proportion of normal chromatin compared to the control and 1 mM groups on day 5 of storage (71.1%–73.8% in control and 1 mM vs. 77.5%–80.8% in 0.25–0.75 mM EP, p<0.01 and p<0.001; Fig. 4). Notably, sperm stored with 0.5 mM EP showed the highest proportion of normal chromatin on days 3 and 5 (p<0.001; Fig. 4).

A similar trend was observed in the ROS levels. On day 1, the ROS levels did not significantly differ among the treatment groups (Fig. 4). The ROS levels were significantly lower in sperm treated with 0.25–0.75 mM EP compared to the control and 1 mM EP on day 3 of storage (fluorescent intensity; 27.9–28.5 in control and 1 mM vs. 17.8-21.9 in 0.25 mM EP, p<0.05, p<0.01 and p<0.001; Fig. 5). A similar trend was seen on day 5, in sperm stored with 0.25–0.75 mM EP (27.9–28.5 in control and 1 mM vs. 17.8–21.9 in 0.25 mM EP, p<0.05, p<0.01 and p<0.001; Fig. 5).

DISCUSSION
Boar spermatozoa are susceptible to oxidative stress due to the high levels of polyunsaturated fatty acids in their cell membranes and lower natural antioxidant defense capacity [23, 24]. As a result, boar spermatozoa are prone to reduced sperm motility, viability, and chromatin stability, along with increased ROS levels during liquid storage [25]. To reduce these effects, antioxidants are added to semen extenders to help maintain the quality of boar spermatozoa by decreasing excessive ROS production during liquid storage at 17°C [26].
AI is widely used as a successful breeding method in boars and improves fertility, genetics, and production. Liquid preservation of boar semen is a crucial component of AI [27]. Therefore, adding antioxidants to semen extenders is essential during storage to maintain sperm quality [28]. Research studies have used both synthetic and natural antioxidants, in vivo and in vitro, to improve boar sperm quality by reducing ROS production during storage [29]. Synthetic antioxidants that have been used include α-tocopherol, butylated hydroxytoluene, taurine, and selenium, while natural extracts such as rosmarinic acid, resveratrol, and Isatis root polysaccharide have also been tested [30].
EP is an ester formed by the condensation of pyruvic acid and can be used as a stable alternative to pyruvate. Researchers have demonstrated that EP has shown antioxidant potential in various cells and tissues, including the testicular tissue in mammals in in vivo studies [31]. Pyruvate is an essential component of the metabolic pathways in cells. It is the end product of anaerobic glycolysis and serves as the starting substrate for the tricarboxylic acid cycle [32]. Both in vitro [33] and in vivo [34] research studies have found that EP is involved in metabolic activities by increasing ATP production and reducing oxidative stress in cells without breaking down into pyruvate.
Several in vivo studies have documented that EP has a protective effect on male reproductive functions. For example, EP administration to mice with cyclophosphamide-induced oxidative stress significantly improved epididymal sperm quality by enhancing sperm concentration, motility, viability, nucleus maturity, and chromatin stability, and led to lower ROS levels compared to cyclophosphamide-induced mice without EP treatment [11]. Similar approach where methotrexate was administered to mice to induce testicular toxicity, followed by treatment with EP showed increased sperm count, motility, viability, sperm maturity, and chromatin stability compared to the mice treated only with methotrexate [35]. Also, mice with PHZ-induced hemolytic anemia were treated with EP and found to reduce the DNA damage in sperm [19].
In our study, we found that motility and chromatin stability were increased in boar spermatozoa stored with 0.25–0.75 mM EP compared to the control group on both days 3 and 5 of the storage period. Additionally, our results showed that significantly lower levels of ROS were observed in boar spermatozoa stored at EP concentrations 0.25–0.75 mM during storage days 3 and 5, indicating the antioxidant potential of EP in maintaining sperm quality during liquid storage. Our findings are consistent with previous studies in which EP reduced oxidative stress and improved sperm parameters in mammalian models with testicular toxicity [11, 35].
The improvement in sperm motility observed in our study may be in part due to the metabolic role of pyruvate derivatives. A study using human spermatozoa incubated with exogenous pyruvate and glucose reported elevated motility and capacitation during incubation. The authors revealed that the combined treatment promoted glycolysis, which contributed to the results [36].
Numerous studies have demonstrated that excessive levels of ROS trigger the peroxidation of polyunsaturated fatty acids, leading to the disruption of the normal morphology of sperm, damage to the sperm DNA, and reduction in motility and viability, which are associated with lower success rates seen in in vitro fertilization [11, 37, 38]. According to our findings, reducing oxidative stress is crucial for sperm preservation, and the inclusion of EP in semen extenders can mitigate these negative effects.
In conclusion, our findings indicate that EP is an effective antioxidant for improving the quality of boar sperm during liquid storage. To our knowledge, this is one of the first studies conducted to investigate the in vitro effects of EP on boar semen extenders under short-term storage conditions. Further research is needed to explore its effect during long-term storage and cryopreservation to evaluate its efficacy with other antioxidants for optimal extender formulations.







