ARTICLE
Na, K-ATPase β2 isoform (atp1b2) expressed in the retina of Xenopus
Md. Mahfujur Rahman, Byung-Yong Park*
Author Information & Copyright ▼
College of Veterinary Medicine, Chonbuk National University, Iksan 570-752, Korea
*Corresponding author: Byung-Yong Park, College of Veterinary Medicine, Chonbuk National University, Iksan 570-752, Korea, Tel: +82-63-270-4874, E-mail:
parkb@jbnu.ac.kr
© Research Institute of Veterinary Medicine. This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Nov 21, 2014; Revised: Dec 6, 2014; Accepted: Dec 11, 2014
Abstract
The ubiquitous Na, K-ATPase is a membrane-bound ion pump located in the plasma membrane in all animal cells and plays an essential role in a variety of cellular functions. Studies in several organisms have shown that this protein regulates different aspects of embryonic development and is responsible for the pathogenesis of several human diseases. Na, K-ATPase is an important factor for retinal development, and combinations of the isoforms of each of its subunits are expressed in different cell types and determine its functional properties. In this study, we performed RT-PCR assay to determine temporal expression and in situ hybridization to determine spatial expression of Na, K-ATPase β2 isoform (atp1b2) in Xenopus laevis. Focusing on retinal expression to distinguish the specific expression domain, we used retinal marker genes sox4, sox11, vsx1, and pax6. Xenopus atp1b2 was expressed from late gastrulation to the tadpole stage. Using whole mount in situ hybridization, we showed that Xenopus atp1b2 was expressed broadly in the eye, the whole surface ectoderm, and gills. In situ hybridization on sections revealed detailed and specific expression in the outer nuclear layer of the retina, which consists of two major classes of photoreceptors, rods and cones, surface ectoderm, pharyngeal epithelium, and gills. These findings indicate that atp1b2 may play an important role for the development of Xenopus retina.
Keywords: Na; K-ATPase β2 isoform; outer nuclear layer; retina; pharyngeal epithelium; Xenopus
Introduction
The vertebrate retina is a light-sensitive layer of tissue, lining the inner surface of the eye. It’s a model for studying mechanism underlying neural cell proliferation, fate choice, signaling and tissue patterning due to its accessibility and simplicity. The retina has six major classes of neurons and a single class of glial cells. The outer nuclear layer consists of cell bodies of rods and cones. The inner nuclear layer contains the Muller cells, horizontal, bipolar and amacrine interneurons. The ganglion cell layer contains nuclei of ganglion cells, the axons of which become the optic nerve fibers for messages and some displaced amacrine cells. The ganglion cells send their axons through the optic nerve to the brain. In amphibians, the site of continuous neurogenesis is in the periphery of the retina and allowing it to grow through the lifespan of the animals [1, 2].
In the retina the Na, K-ATPase restores Na+ and K+ gradients used by the photoreceptor dark current, synaptic activity, action potentials, and transmitter uptake. Inner segments of the outer nuclear layer of retina have the high concentrations of Na, K-ATPase [3]. The ubiquitous Na, K-ATPase is a membrane bound ion pump located in the plasma membrane in all animal cells, where it maintains the electrochemical gradients of sodium and potassium ion across the membranes and plays an essential role for variety of cellular functions including osmoregulation, sodium coupled transport of variety of organic molecules, neuronal and muscle cells activity. The Na, K-ATPase is composed of two non-covalently linked subunits: catalytic α subunit as well as β subunit which is required for the structural and functional maturation of α subunit [4-11].
The β subunit is a type II glycosylated membrane protein required for the modulation of sodium and potassium ion of the functional enzyme [12, 13]. In mice, deficiency of β2 (atp1b2) exerts motor incoordination, tremors and paralysis of the extremities [14]. The atp1b2 expression abrogates glioblastoma-derived brain tumor-initiating cells in human [15]. Genetically modified atp1b2 subunits are associated with apoptosis of photoreceptors in mice [14, 16, 17]. In postnatal mouse, increases in atp1b2 expression in bipolar cells occur very late, coinciding with synaptogenesis in the inner plexiform layer [18]. Here, we report the temporal and detailed expression analysis of atp1b2 during Xenopus embryogenesis.
Materials and Methods
Xenopus laevis Husbandry
Xenopus laevis was handled in accordance with animal welfare regulations of Institutional Animal Care and Use Committees (IACUC), Chonbuk National University Laboratory Animal Centre, South Korea. Xenopus laevis embryos were maintained according to standard protocols. All efforts were made to minimize the discomfort of animal used.
Reverse Transcriptase Polymerase Chain Reaction
Total RNA was extracted and digested with DNase I and purified with RNeasy cleanup kit (Qiagen). First strand cDNA was synthesized from stage 35 embryos with RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). RT-PCR was performed by using Maxim RT-PCR Premix Kit (iNtRON). The partial sequences of the X. laevis atp1b2, vsx1 and pax6 ORF were amplified using PCR from stage 30 cDNA using a set of primers designed against two conserved domain regions of each gene in several species. The resulting PCR products of 1,018 bp for atp1b2, 1,002 bp for vsx1 and 1,010 bp for pax6 were purified, subcloned into a pGEM-T Easy vector (Promega), and sequenced. Primers designed for cloning are shown in Table 1. The PCR conditions were 95°C for 30 seconds, 45°C for 30 seconds, 72°C for two minutes for 35 cycles, and final extension at 72°C for 15 minutes.
Table 1.
Primer sequences for cloning and RT-PCR
| Gene Name |
|
Primer Sequence
|
Gene ID |
| Forward |
Reverse |
|
|
atp1b2
|
RT-PCR |
TCGCATCAACAAGTGAAAGC |
TCGCATCAACAAGTGAAAGC |
NM_001086893 |
| Cloning |
CCGTCATCTTCCTCATTGGT |
CCGTCATCTTCCTCATTGGT |
|
vsx1
|
Cloning |
AGCAAAATCAAAGGGCAAGA |
AGCAAAATCAAAGGGCAAGA |
NM_001096722 |
|
pax6
|
Cloning |
GCCACATTCCCATTAGCAGT |
GCCACATTCCCATTAGCAGT |
NM_001085944 |
Download Excel Table
In situ hybridizations
Xenopus laevis eggs were collected, fertilized, and embryos were cultured under standard procedure in amphibian [19]. Anti-sense Digoxigenin-labeled probe was transcribed as the standard procedures [20]. The atp1b2 plasmids were linearized with Spe I and transcribed with T7 polymerase. The retinal marker sox4, sox11 plasmids were linearized with Cla I and EcoR V, respectively, and transcribed with T7 polymerase. The other retinal marker vsx1 and pax6 plasmids were linearized with Sac II and transcribed with SP6 polymerase. Whole mount images were taken on an Olympus MV × 10 microscope.
Stage 27, 30, 35, 41 and 47 embryos were dehydrated with a series of ethanol, transferred to xylene, and then embedded in paraffin. Serial sections of 12 μm were cut with microtome (Thermo, MICROM, HM 325). Anti-sense Digoxigenin-labeled probe was transcribed for in situ hybridization on sections and counterstained with eosin. The images were acquired digitally using a Leica DM 2500 microscope.
Histological analysis
Stage 47 embryos were dehydrated with a series of ethanol, transferred to Xylene, and then embedded in paraffin. Serial sections of 5 μm were cut with microtome and stained with hematoxylin and eosin.
Results
Temporal expression analysis
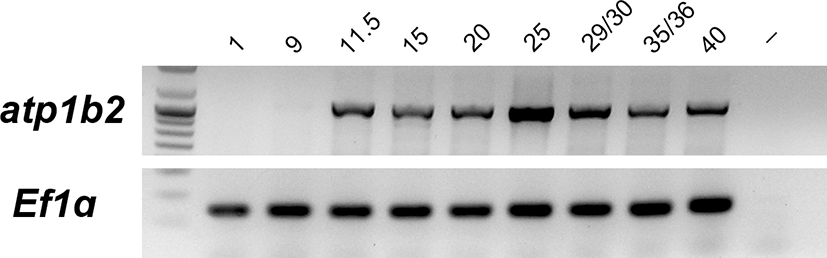
The temporal expression patterns of Xenopus laevis atp1b2 (Fig. 1) were analyzed by RT-PCR using RNA of different developmental stages [21]. The atp1b2 expression, which was not observed during early cleavage stages (stage 1~9), was observed at the late gastrulation stage (stages 11.5) and continued to be present until stage 40.
Fig. 1.
Temporal expression of atp1b2 during Xenopus development. Expression was analyzed by RT-PCR using RNA isolated from embryos at the indicated developmental stages. Elongation factor 1 alpha (Ef1α) was used as an internal control. Lane (-) shows a negative control in absence of RNA.
Download Original Figure
Spatial expression analysis
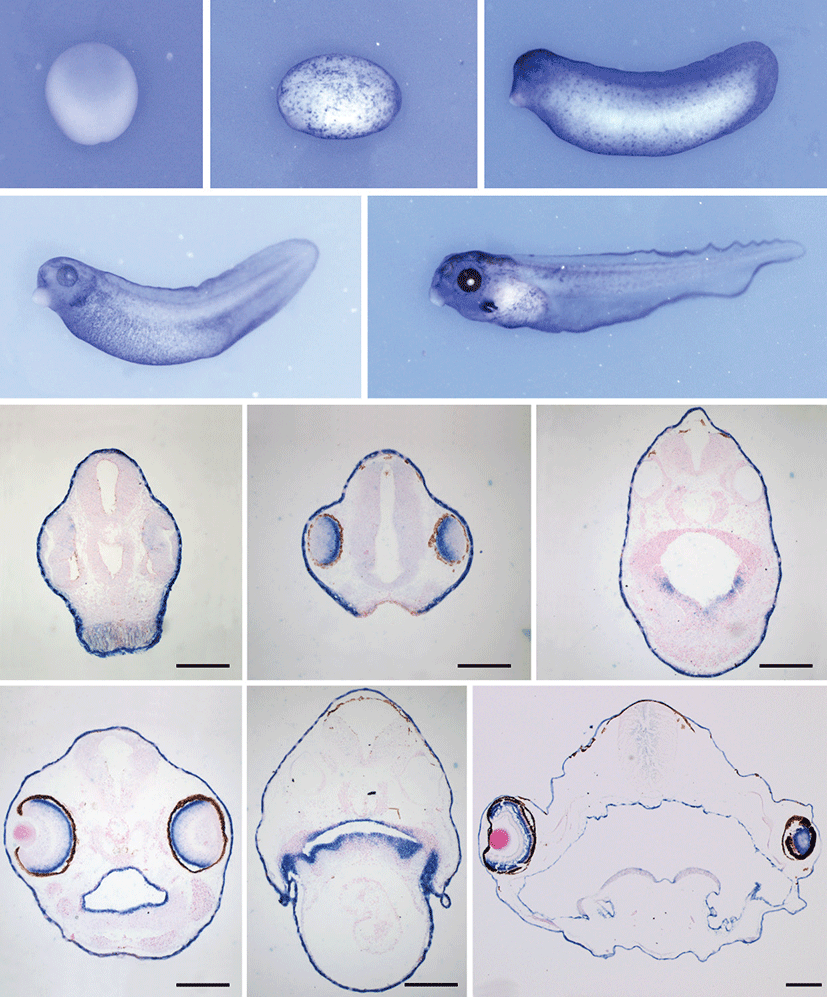
Embryos of stage 15, 20, 30 (tailbud), 40 and stage 45 (tadpole) were used to examine the tissue distribution of Xenopus atp1b2. We were interested in establishing the onset of atp1b2 expression during Xenopus retinal development. The results of in situ hybridization analysis were shown in Fig. 2. Whole mount in situ hybridization showed that in early neurula stage embryos (stage 15) Xenopus atp1b2 was expressed in the whole neuroectoderm (Fig. 2A) and at late neurula stage (stage 20) it was expressed in the whole surface ectoderm (Fig. 2B). At tailbud stage (stage 30) atp1b2 was expressed in the eye and the developing brain with entire epidermal cells (Fig. 2C). Xenopus atp1b2 expression was observed in the developing gills in tadpoles (stage 40 and 45) along with other expression domain (Figs. 2D and 2E). Gene expression persisted at a high level at least until stage 45, the last stage examined in the present study.
Fig. 2.
Developmental expression of atp1b2 by in-situ hybridization. Whole mount in situ hybridization results (A-E). Xenopus atp1b2 was expressed in the neuroectoderm at stage 15 (A), in the entire epidermal cell layer at stage 20 (B), in the surface ectoderm and in the eye and brain at stage 30 (C), and in the brain, eye, developing gills along with surface ectoderm at stage 40 and 45 respectively (D, E). F, G, H, I, J and, K are the results of in situ hybridization on serial sections at different stages. The atp1b2 was expressed in the epidermal cell layer and retina at stage 30 (F) and 35 (G), respectively. It was expressed in the pharyngeal epithelium at stage 35 (H) and in the epidermal cell layer, retina, pharyngeal epithelium along with a projections for developing gills at stage 41 (I, J). The expression of atp1b2 persisted in the brain and specifically in the outer nuclear layer of retina at stage 47 (K). Scale bar=200 μm.
Download Original Figure
Whole mount in situ hybridization revealed that Xenopus atp1b2 was expressed in the eye at stages 30 to 45. To determine the specific layer in which atp1b2 was expressed, we performed in situ hybridization on the serial sections of stage 30, 35, 41 and 47 embryos. At tailbud stage (stage 30) Xenopus atp1b2 was expressed in the retina and distinguishingly in the epidermal cells (Fig. 2F). At stage 35 we found an exclusive expression domain that was pharyngeal epithelium (Figs. 2G and 2H) and its expression persisting as the same pattern at stage 41 including the lateral prominence for gills (Figs. 2I and 2J). In tadpole (stage 47), the expression of almost the same domains including neural tube and the future brain also persisted, but at this stage the retinal expression was more specific in the outer nuclear layer of retina (Fig. 2K).
Na, K ATPase β2 subunit expression in the retina of eye
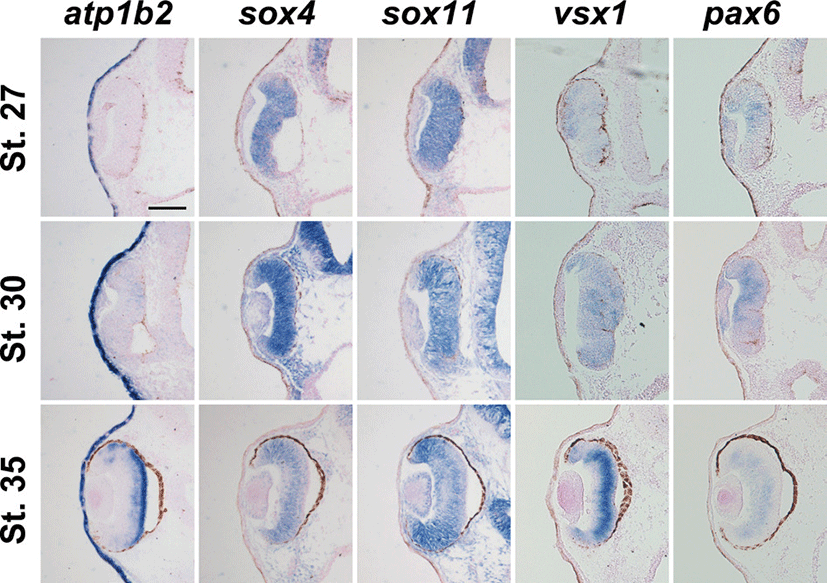
We also performed in situ hybridization on sections with different proneural and panneural retinal cell markers. We used sox4 and sox11 to visualize ganglion cell layer, pax6 to visualize amacrine and ganglion cells, vsx1 to detect bipolar cells and compare the expression with atp1b2. After conducting in situ hybridization on sections, it was observed that atp1b2 was expressed in the retina at stage 27, in contrast to atp1b2, and all other marker genes were expressed in the retina at this stage (Figs. 3A-E). Xenopus atp1b2, vsx1, and pax6 were weakly expressed in the retina at stage 30 whereas sox4 and sox11 were strongly expressed (Figs. 3F-J). Since Xenopus atp1b2 was expressed in the retina we have sectioned later stage to see the specific layer of expression. At stage 35, atp1b2 was expressed in outer part of the retina and among the retinal marker genes sox4 and sox11 were expressed strongly in the ciliary marginal zone (CMZ) whereas pax6 was not expressed in the CMZ (Figs. 3K-O).
Fig. 3.
Expression analysis of atp1b2 in the retinal development before cellular differentiation. Transverse sections were performed at three different stages along the anteroposterior axis. Each row shows sections of different stages embryos and each column shows expression of different marker genes for retinal development. Expression of atp1b2, sox4, sox11, vsx1 and pax6 at stage 27 (A-E), at stage 30 (F-J), at stage 35 (K-O) respectively. Scale bar=100 μm.
Download Original Figure
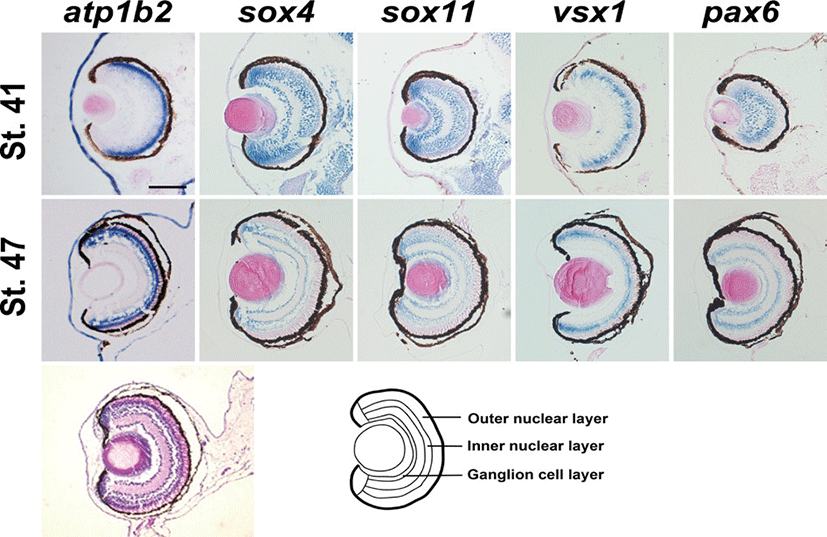
The specific retinal layer was not clearly detectable at stage 41 but we found the different domain of expressions (Figs. 4A-E). At stage 47, Xenopus atp1b2 was specifically expressed in the outer nuclear layer of retina whereas sox4 and sox11 in the inner nuclear layer and ganglion cell layer, vsx1 in the inner nuclear layer, and pax6 were expressed both in the inner nuclear layer and ganglion cell layer (Figs. 4F-J).
Fig. 4.
Expression analysis of atp1b2 in the retinal development after cellular differentiation. Expression of Xenopus atp1b2 in the retina at stage 41 (A-E) and at stag 47 (F-J), hematoxylin and eosin stained embryos (stage 47) showing different layer of retina (K), and diagram of eye showing different layer of retina (L). Scale bar=100 μm.
Download Original Figure
Discussion
The atp1b2 was first cloned as an adhesion protein and there is some evidence for its mediation of cell–cell interactions [14]. The question arises whether it could play a role in cell adhesion and histogenesis. Its only early expression in the mouse retina, however, was in photoreceptors that have already taken up position scleral to the still proliferating progenitor cells [22, 23]. Its expression in bipolar cells occurred long after histogenesis and appeared to reflect the increased need for ion transport consequent to synaptogenesis [16]. Investigation of the retina thus supports a role for atp1b2 only in Na, K-ATPase activity.
In this study, we focused that atp1b2 is expressed in the outer nuclear layer of retina in developing Xenopus embryo. We here provide a detailed description of the tissue specific expression of atp1b2 isoform during Xenopus embryogenesis and thereby extend earlier finding by others.
The vertebrate retina consists of three nuclear layers of retina, the outer nuclear layer, the inner nuclear layer, and ganglion cell layer. Among them the outer nuclear layer consists of two major classes of photoreceptors, rods and cones [24]. Our data showed the expression of atp1b2 in the outer nuclear layer. To detect the specific layer of expression we also observed the expression of other retinal marker genes, where sox4 and sox11 were expressed in the inner nuclear layer and ganglion cell layer [25], pax6 was expressed in the inner nuclear layer and ganglion cell layer [26], and vsx1 was expressed in the inner nuclear layer of retina [27]. The pax6 expression was also devoid of the mature cells of the outer half of the retina and CMZ [28]. Tissue specific expression was summarized in Table 2. It is notable that in some cell types, elevated levels of particular Na, K-ATPase subunit isoforms (atp1a3 with atp1b2 in photoreceptors) preceded significantly other phenotypic differentiation. In most other cases, however, detectable expression coincided with the adoption of differentiated characteristics and upregulation corresponded with increases in retinal function.
Table 2.
Tissue-specific expression of atp1b2 along with other marker in the retina during Xenopus development
| Tissue Specified |
atp1b2 |
atp1b2 |
sox11 |
vsx1 |
pax6 |
|
| Ciliary Marginal Zone (CMZ) |
+ |
- |
- |
- |
- |
| Retinal pigment epithelium |
- |
- |
- |
- |
- |
| Outer nuclear layer |
+ |
- |
- |
- |
- |
| Inner nuclear layer |
- |
+ |
+ |
+ |
+ |
| Ganglion cell layer |
- |
+ |
- |
- |
+ |
Download Excel Table
Numerous studies have now confirmed that Na, K-ATPase subunit isoform composition has effects on the affinities of the enzyme for Na+, K+, and ouabain [3, 29]. Substitution of atp1b2 or atp1b3 for atp1b1 (as occurs in photoreceptors) increases affinity for Na+ in Sf-9 cells [3, 30] but may have different effects in other cells with other isoforms [31, 32].
We also observed the expression of atp1b2 in the pharyngeal epithelium. The homologues of atp1b2 have also been confirmed in some other vertebrates [33, 34]. Along with these expression atp1b2 was also expressed in the developing brain, developing gills, and whole epidermal cells.
The overall conclusion of the present study is that the atp1b2 may have an important role for the development of outer nuclear layer of retina in developing Xenopus. However, further studies will be necessary to demonstrate a direct role of atp1b2 in the retinal development along with its other expression domain.
Acknowledgements
This study was financially supported by grants from the Korea Research Foundation Grant (2011-0014454). We are grateful to Dr. Susanne Kuhl, Institute of Biochemistry and Molecular Biology, Ulm University, Ulm, Germany for giving sox4 and sox11 plasmids.
REFERENCES
Casarosa S, Leone P, Cannata S, Santini F, Pinchera A, Barsacchi G, Andreazzoli M. Genetic analysis of meta-morphic and premetamorphic Xenopus ciliary marginal zone. Dev Dyn. 2005; 233 p. 646-651.

Perron M, Kanekar S, Vetter ML, Harris WA. The ge¬netic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998; 199 p. 185-200.

Blanco G, Mercer RW. Isozymes of the Na-K-ATPase heterogeneity in structure, diversity in function. Am J Physiol. 1998; 275 p. F633-F650.

Crambert G, Schaer D, Roy S, Geering K. New molecu¬lar determinants controlling the accessibility of ouabain to its binding site in human Na,K-ATPase alpha iso¬forms. Mol Pharmacol. 2004; 65 p. 335-341.

Eid SR, Brandli AW. Xenopus Na,K-ATPase primary sequence of the beta2 subunit and in situ localization of alpha1, beta1, and gamma expression during proneph¬ric kidney development. Differentiation. 2001; 68 p. 115-125.

Gloor S. Cloning and nucleotide sequence of the mouse Na,K-ATPase beta-subunit. Nucleic Acids Res. 1989; 17 p. 10117-0.

Henriksen C, Kjaer-Sorensen K, Einholm AP, Madsen LB, Momeni J, Bendixen C, Oxvig C, Vilsen B, Larsen K. Molecular cloning and characterization of porcine Na(+)/K(+)-ATPase isoforms alpha1, alpha2, alpha3 and the ATP1A3 promoter. PLoS One. 2013; 8 p. e79127-0.

Herrera VL, Emanuel JR, Ruiz-Opazo N, Levenson R, Nadal-Ginard B. Three differentially expressed Na,K-ATPase alpha subunit isoforms structural and func¬tional implications. J Cell Biol. 1987; 105 p. 1855-1865.

Malik N, Canfield VA, Beckers MC, Gros P, Levenson R. Identification of the mammalian Na,K-ATPase 3 subunit. J Biol Chem. 1996; 271 p. 22754-22758.

Mercer RW, Schneider JW, Savitz A, Emanuel J, Benz EJ, Levenson R. Rat-brain Na,K-ATPase beta-chain gene: primary structure, tissue-specific expression, and amplification in ouabain-resistant HeLa C+ cells. Mol Cell Biol. 1986; 6 p. 3884-3890.

Messenger NJ, Warner AE. Primary neuronal differen¬tiation in Xenopus embryos is linked to the beta(3): sub¬unit of the sodium pump. Dev Biol. 2000; 220 p. 168-182.

Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr. 2001; 33 p. 425-438.

Geering K, Beggah A, Good P, Girardet S, Roy S, Schaer D, Jaunin P. Oligomerization and maturation of Na,K-ATPase functional interaction of the cytoplasmic NH2 terminus of the beta subunit with the alpha sub-unit. J Cell Biol. 1996; 133 p. 1193-1204.

Magyar JP, Bartsch U, Wang ZQ, Howells N, Aguzzi A, Wagner EF, Schachner M. Degeneration of neural cells in the central nervous system of mice deficient in the gene for the adhesion molecule on Glia, the beta 2 sub¬unit of murine Na,K-ATPase. J Cell Biol. 1994; 127 p. 835-845.

Sun MZ, Kim JM, Oh MC, Safaee M, Kaur G, Clark AJ, Bloch O, Ivan ME, Kaur R, Oh T, Fouse SD, Phil¬lips JJ, Berger MS, Parsa AT. Na(+)/K(+)-ATPase beta2-subunit (AMOG) expression abrogates invasion of glioblastoma-derived brain tumor-initiating cells. Neuro Oncol. 2013; 15 p. 1518-1531.

Molthagen M, Schachner M, Bartsch U. Apoptotic cell death of photoreceptor cells in mice deficient for the adhesion molecule on glia (AMOG, the beta 2- subunit of the Na, K-ATPase). J Neurocytol. 1996; 25 p. 243-255.

Weber P, Bartsch U, Schachner M, Montag D. Na,K-ATPase subunit beta1 knock-in prevents lethality of beta2 deficiency in mice. J Neurosci. 1998; 18 p. 9192-9203.

Wetzel RK, Arystarkhova E, Sweadner KJ. Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci. 1999; 19 p. 9878-9889.

Batut J, Neant I, Leclerc C, Moreau M. xMLP is an early response calcium target gene in neural determina¬tion in Xenopus laevis. Journal de la Societe de biologie. 2003; 197 p. 283-289.

Jones CM, Smith JC. Mesoderm induction assays. Methods Mol Biol. 2008; 461 p. 395-404.

Nieuwkoop PD, Faber J. Normal table of Xenopus lae¬vis (Daudin): a symmetrical and chronological survey of the development from the fertilized egg till the end of metamorphosis. 19941st edNew York & LondonGarland Publishing Inc.

Hicks D, Barnstable CJ. Different rhodopsin monoclo¬nal antibodies reveal different binding patterns on de-veloping and adult rat retina. J Histochem Cytochem. 1987; 35 p. 1317-1328.

Jasoni CL, Reh TA. Temporal and spatial pattern of MASH-1 expression in the developing rat retina dem-onstrates progenitor cell heterogeneity. J Comp Neurol. 1996; 369 p. 319-327.

Parain K, Mazurier N, Bronchain O, Borday C, Cabo¬chette P, Chesneau A, Colozza G, El Yakoubi W, Ham-dache J, Locker M, Gilchrist MJ, Pollet N, Perron M. A large scale screen for neural stem cell markers in Xeno-pus retina. Dev Neurobiol. 2012; 72 p. 491-506.

Jiang Y, Ding Q, Xie X, Libby RT, Lefebvre V, Gan L. Transcription factors SOX4 and SOX11 function re-dundantly to regulate the development of mouse retinal ganglion cells. J Biol Chem. 2013; 288 p. 18429-18438.

Hitchcock PF, Macdonald RE, VanDeRyt JT, Wilson SW. Antibodies against Pax6 immunostain amacrine and ganglion cells and neuronal progenitors, but not rod precursors, in the normal and regenerating retina of the goldfish. J Neurobiol. 1996; 29 p. 399-413.

Hayashi T, Huang J, Deeb SS. RINX(VSX1), a novel homeobox gene expressed in the inner nuclear layer of the adult retina. Genomics. 2000; 67 p. 128-139.

Hirsch N, Harris WA. Xenopus Pax-6 and retinal devel¬opment. J Neurobiol. 1997; 32 p. 45-61.

Sweadner KJ. Isozymes of the Na+/K+-ATPase. Bio chim Biophys Acta. 1989; 988 p. 185-220.

Yu C, Xie Z, Askari A, Modyanov NN. Enzymatic properties of human Na,K-ATPase alpha1beta3 iso¬zyme. Arch Biochem Biophys. 1997; 345 p. 143-149.

Jaisser F, Jaunin P, Geering K, Rossier BC, Horisberger JD. Modulation of the Na,K-pump function by beta subunit isoforms. J Gen Physiol. 1994; 103 p. 605-623.

Schmalzing G, Kroner S, Schachner M, Gloor S. The adhesion molecule on glia (AMOG/beta 2): and alpha 1 subunits assemble to functional sodium pumps in Xen¬opus oocytes. J Biol Chem. 1992; 267 p. 20212-20216.

Carmosino M, Torretta S, Procino G, Timperio A, Zolla L, Svelto M. Na+/K+-ATPase beta1-subunit is recruit¬ed in Na-K-2Cl co-transporter isoform 2 multiprotein complexes in rat kidneys possible role in blood pres¬sure regulation. J Hypertens. 2014; 32 p. 1842-1853.

Tokhtaeva E, Sachs G, Vagin O. Diverse pathways for maturation of the Na,K-ATPase beta1 and beta2 sub¬units in the endoplasmic reticulum of Madin-Darby ca¬nine kidney cells. J Biol Chem. 2010; 285 p. 39289-39302.