Introduction
Brucellosis is a major zoonosis in domestic animals and abortuscauses economic losses for the livestock industry as well as public health problems for humans [27]. The disease is caused by Brucella spp., which are small, Gram-negative, non-motile and polymorphic rods. Brucella spp. are facultative intracellular parasites that cause abortion and infertility in various mammals and the well-known undulant fever in humans [9]. The bacteria penetrate the mucosa of the nasal, oral, or pharyngeal cavities and are phagocytized by host macrophages, where survival and replication occurs [12].
Currently, combinations of two or three antibiotics are used to treat brucellosis due to the low efficacy of monotherapy. Several clinical studies analyzed the efficiency of different antibiotic regimens, and identified problems such as financial considerations in developing countries, therapeutic failures, relapses and the emergence of drug resistance [4, 32].
In general, tetracycline/aminoglycoside combinations are the most common antibiotics used in the treatment of brucellosis [31]. However, due to high rates of treatment failure or relapses due to emerging drug resistance, antibiotic treatment of brucellosis is still problematic [24]. Thus, alternative therapies to treat brucellosis are needed.
Conventional herbal medicines have long been used as remedies against infectious diseases in Asian countries. Galla rhois (GR) has long been used in traditional Asian medicine to treat diarrhea, persistent coughing and spontaneous perspiration due to its antidiarrhetic, astringent and hemostatic properties [10, 13, 22, 23]. GR is a harmless natural material that contains a number of tannin-derived components, including methyl gallate and gallic acid [15]. Notably, the gallotannins are a class of hydrolysable tannin polymers formed from gallic acid, which seems to have anti-bacterial, anti-fungal, and anti-viral properties [3, 14]. In a previous study [22], GR ethanol extract exhibited antibacterial and protective activities against Brucella abortus (B. abortus) in vitro and in vivo.
Sodium chlorate (SC) is used as an oxidizing agent and for making chlorine dioxide used in water disinfection. Chlorate is found as a stable by-product in drinking water that has been disinfected with chlorine dioxide [25]. Previous studies have been carried out on the prevention and treatment of Enterobacteriaceae infections in animals using SC [6, 7]. B. abortus like Salmonella species has a respiratory nitrate reductase enzyme, which coincidentally catalyses the intracellular reduction of chlorate into chlorite, a cytotoxic product that kills the bacterium in tissue cells [8, 11]. As most of the normal anaerobic gut bacteria lack respiratory nitrate reductase activity, chlorate selectively targets bacteria expressing respiratory nitrate reductase activity but not beneficial anaerobes lacking that enzyme [6, 7].
Although previous studies [6-8, 22] investigated the antibacterial effects of each of GR and SC, few studies have been carried out to investigate the effect of a combination of GR and SC on mice infected with B. abortus. The present study evaluated the therapeutic potential of a combination of GR ethanol extract (GRE) and SC on murine brucellosis.
Materials and Methods
GR powder was obtained from GS Bio (Jeonju, Korea), isolated from plant material and analyzed as described previously [2, 22]. Briefly, 1 kg of plant material was dried in an oven at 60°C for 3 days and extracted with ethanol twice at room temperature. The remaining residue was removed by filtration (Whatman no. 2, Sigma-Aldrich Korea, Yonin), and the filtrate was concentrated using a vacuum rotary evaporator (Iwai Co., Japan), followed by freezing of the dried powder. This crude, extracted powder was used in the present study.
SC was purchased from Sigma-Aldrich Korea (Yonin, Korea). To make a 30 mM SC stock solution, 3.19 g of SC was dissolved in distilled water up to a final volume of 1L. After the SC stock solution was suitably diluted with distilled water, the diluents were used in this study.
Six-week-old specific pathogen-free (SPF) female ICR mice were obtained from Samtaco Co. (Osan, Korea). All animals were kept at the inspecting facility of Gyeongsang National University (Chinju, Korea) for 1 week to allow acclimation before experimentation. Thereafter, they were kept in an isolated SPF barrier room with regulated temperature (23 ± 1°C), humidity (50 ± 5%) and light/dark cycle (12/12 hr). The animals were fed a sterilized (2 M rad radiation) pellet diet (Purina, Seoul, Korea) and sterilized water ad libitum. All studies were performed in accordance with the Guide for Animal Experimentation of Gyeongsang National University and approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (Approval No. GNU-2013-12-10). All efforts were made to minimize pain or discomfort experienced by the used animals.
B. abortus strains were derived from 544 (ATCC 23448), a smooth, virulent B. abortus biovar 1 strain. B. abortus strains were maintained as frozen glycerol stocks and were cultured in Brucella broth (Becton Dickinson, Sparks, MD.) or Brucella broth containing 1.5% agar without antibiotics for 3 days at 37°C. Bacteria were grown at 37°C with vigorous shaking until they reached the stationary phase, and bacterial growth rates were measured using a spectrophotometer (Beckman Coulter Korea, Seoul) at a wavelength of 600 nm.
After a 1-week adaptation period, all mice were injected intraperitoneally with 2 × 104 CFUs of B. abortus. Similar to previous studies [6-8, 22], forty ICR mice infected with B. abortus were randomly divided into four groups: PC (Positive control), GRT (GRE 200 mg/L drinking water), SCT (sodium chlorate 1.6 g/L drinking water), and GST (GRE 200 mg + SC 1.6 g/L drinking water). All mice were fed drinking water treated with each drug ad libitum for 14 days.
On day 7 and 14 after drug treatment, five mice from each group were sacrificed, and their bloods were collected, and centrifuged at 1,000 × g for 30 min to separate the serum. According to previous protocols for the tube agglutination test (TAT) [26, 29], the diagnostic antigen for B. abortus, supplied by the Animal and Plant Quarantine Agency (Anyang, Korea), was diluted at 1:100 in phenol saline before use. Thereafter, 0.08, 0.04, 0.02, 0.01, and 0.005 mL of serum samples, inactivated at 56°C for 30 min, were placed in different tubes and mixed with 2 mL of the diluted antigen. The results were read after incubation at 37°C for 48 hr. The criteria for positive, suspected positive and negative reactions were greater than 100, 50, and less than 25 serum dilution, respectively [20, 33].
On day 7 and 14 post-infection, five mice from each group were sacrificed, and their spleens were removed and weighed. In addition, each spleen was homogenized in PBS. Continually, the homogenates were serially diluted with PBS and plated on Brucella agar. After the plates were incubated at 37°C for 3 days, the number of CFUs in each spleen was counted and represented as CFU/g spleen.
At day 14 post-infection, five mice from each group were sacrificed, and their livers were removed. The livers were fixed in 10% neutral-buffered formalin for at least 24 hr. Tissues were dehydrated in graded alcohols, cleared with xylene, and infiltrated and embedded in paraffin. Tissues embedded in paraffin were cut to a thickness of 4~6 μm and mounted on glass slides. The sections were stained with hematoxylin and eosin (H&E) and examined for histopathological changes under a light microscope (Olympus, Tokyo, Japan).
Results
To identify the inhibitory effects of GRE, SC and a mixture of GRE and SC, indicated by a decrease in the ability of B. abortus to promote infection within the host, mice infected with B. abortus were treated with GRE, SC and a mixture of GRE and SC, and serum antibodies of B. abortus were titered at day 7 and 14 post-infection.
The reciprocal antibody titers from TAT in sera for the ICR mice infected with B. abortus are presented in Table 1. At 7 days post-infection, the antibody titers in PC and SCT ranged from 1:50 to 1:100≤ and those in GRT and GST ranged from ≤1:25 to 1:100≤. At 14 days post-infection, the antibody titers ranged from 1:200 to 1:400 in PC, from ≤1:25 to 1:100≤ in SCT and from ≤1:25 to 1:50 in GRT and GST. In GRT and GST at 7 and 14 days post-infection, the reciprocal antibody titers showed a tendency to decrease compared to NC.
| Group | After treatment | Negative |
Suspected positive |
Positive |
||
|---|---|---|---|---|---|---|
| ≤1:25 | 1:50 | 1:100≤ | 1:200 | 1:400 | ||
| PC | Day 7 | 2 | 3 | |||
| Day 14 | 1 | 4 | ||||
| SCT | Day 7 | 2 | 3 | |||
| Day 14 | 2 | 2 | 1 | |||
| GRT | Day 7 | 1 | 2 | 2 | ||
| Day 14 | 3 | 2 | ||||
| GST | Day 7 | 1 | 2 | 2 | ||
| Day 14 | 4 | 1 | ||||
The average spleen weight in PC, GRT, SCT and GST was 0.25 ± 0.05 g, 0.18 ± 0.04 g, 0.21 ± 0.04 g and 0.16 ± 0.03 g, respectively. In GRT, SCT and GST at 14 days post-infection, the average spleen weight decreased compared to those in PC (Table 2). However, there was no significant difference between groups.
| Item | Group | |||
|---|---|---|---|---|
| PC | SCT | GRT | GST | |
| Weight (g) | 0.25 ± 0.05 | 0.21 ± 0.04 | 0.18 ± 0.04 | 0.16 ± 0.03 |
Fig. 1 shows the number of bacteria recovered from the spleens in each group at 7 and 14 days post-infection. At 7 days post-infection, there was a significant difference between PC and GST (P<0.05). At 14 days post-infection, bacterial numbers in all treated groups were significantly decreased compared to PC (GRT and SCT, P<0.05; GST, P<0.001). These findings indicate that GRE, SC and a combination of GRE and SC have a therapeutic effect on murine brucellosis, and the mixture of GRE and SC shows the most curative effect.
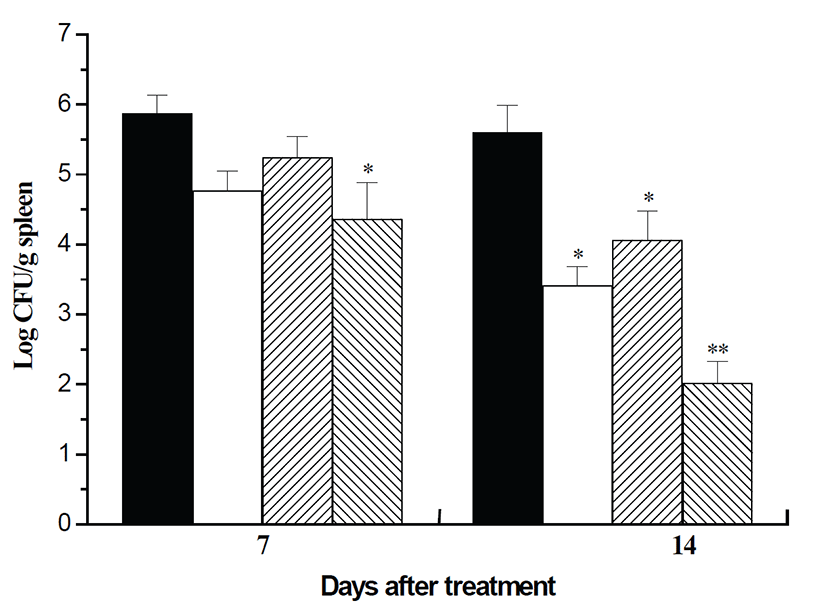
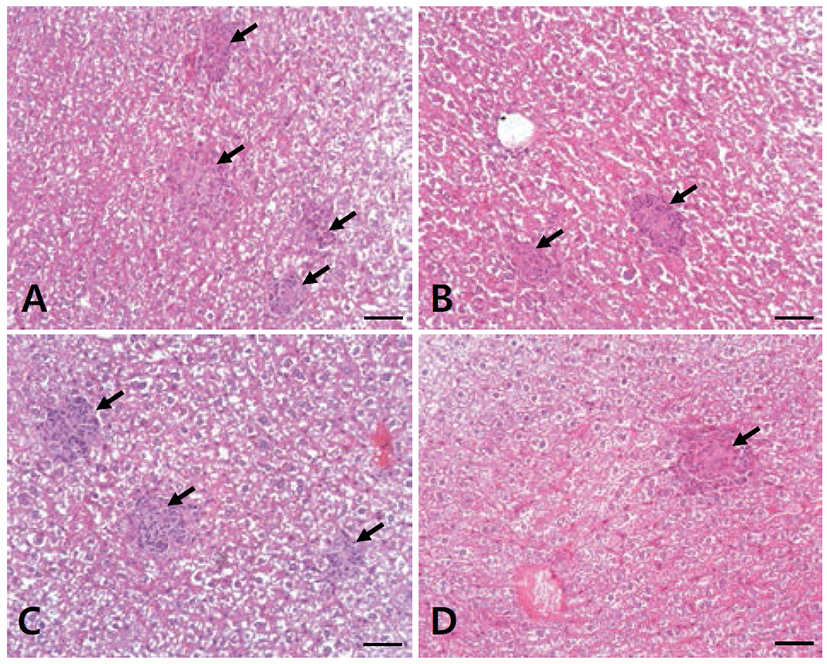
To examine the pathologic changes from the formation of microgranulomas containing inflammatory cells, slides were prepared from the livers of mice infected with B. abortus, and observed under a light microscope. In the mice livers, there were numerous multifocal microgranulomas in PC, and their number decreased successively in SCT, GRT and GST (Fig. 1).
Discussion
Brucellosis is one of the world’s most widespread zoonoses and is of major economic importance in most countries around the world [5]. The disease has been efficiently controlled in agricultural animals through the use of vaccination, surveillance and confined programs [22]. However, there is currently no safe or efficient vaccine that can be recommended to control brucellosis [19]. In addition, treatment of the disease with antibiotics remains controversial and requires prolonged therapy with at least two agents [28]. Moreover, Brucella organisms escape the host’s defense system, as the bacteria invade, resist killing, replicate and appear to be well-adapted to endure the multiple environmental conditions in an intracellular niche inside the host [21]. Therefore, conventional antibiotic regimens are not suitable for the treatment of Brucella infection due to its escape from the host’s defense system and the rapid emergence of antibiotic resistance [1].
In previous studies, research on alternatives to conventional antibiotics to treat brucellosis using natural products has not been widely reported. This study was carried out to determine the potential of GRE, SC and a combination of GRE and SC as alternatives to antibiotics for brucellosis treatment.
In this study, the negative results of serum antibody titers in GRT and GST were 60% (3/5) and 80% (4/5) after 14-day treatment, respectively, while the positive result of those in NC was 100% at 14 days post-infection. In the antibiotic treatment of canine brucellosis, all infected dogs given 5 mg/kg of enrofloxacin orally every 12 hr for 30 days were reported negative on the serological rapid slide agglutination test [34]. In addition, the results of antibiotic treatment for 6 weeks in patients with brucellosis were reported positive in 20% of patients by serum plate agglutination test (SPA), while the SPA results were positive in 80% of patients before antibiotic treatment [16]. Considering the infected subjects and the treatment period, GRE and a combination of GRE and SC in this study were more therapeutic than the antibiotics in the above studies.
The liver is an important site for colonization and replication of Brucella in mice. Usually, mice infected with virulent strains of Brucella spp. have mild to moderate hepatitis, which is characterized by neutrophilic infiltrate in the early stages of infection, followed by histiocytic infiltrate with epithelioid cells and microgranulomas in the chronic stages of infection [18, 30]. In this study, microgranulomas were found in the liver of mice infected with B. abortus in NC at 14 days post-infection. In addition, microgranulomas gradually decreased in SCT, GRT and GST, which indicate that SC, GRE and a combination of GRE and SC have a therapeutic effect on murine brucellosis. Fig. 2.
To examine the effects of the treatment of SC, GRE and a mixture of GRE and SC, mice were infected with B. abortus. In SCT, GRT and GST, the average weight of spleens collected from infected mice showed no significant difference compared to those in PC. However, the number of B. abortus in the spleens in GRT (P<0.05) and GST (P<0.001) was significantly decreased compared to that in PC, and the bacterial loads in the spleens of mice in GRT and GST after 14 days post-treatment were more than 150- and 3,000-fold lower than those of PC, respectively (Fig. 1). These results indicated that a combination of GRE and SC has a potential therapeutic effect on murine brucellosis. In a previous study on the treatment of murine brucellosis with antibiotics [17], erythromycin was orally administered to infected mice at a concentration of 200 mg/kg/day for 15 days post-infection, and the number of B. abortus recovered from the spleens of the treated mice was approximately 16-fold lower than that in the untreated mice. Taking into account the bacteria decrease in the spleens of mice, GRE and the combination of GRE and SC in this study have a greater potential therapeutic effect than erythromycin.
Conclusively, this study emphasizes the idea that the combination of GRE and SC is effective in the treatment of brucellosis, and possibly other diseases caused by intracellular pathogenic bacteria, and is an alternative to conventional therapy regimes.
In the future, it will be necessary to determine the clinical usefulness of a mixture of GRE and SC in the treatment of brucellosis.







