INTRODUCTION
The global prevalence of obesity has driven the development of various treatment strategies aimed at improving weight loss and metabolic health [1]. Although these approaches have considerably advanced, existing therapies may cause adverse side effects or pose the risk of weight regain [2]. Therefore, safer, more effective, and sustainable alternatives, such as plant-based treatments, should be established to manage obesity. Plant-based natural products have gained considerable attention as novel therapeutic agents because of their bioactive properties and minimal side effects [3].
Herbal compounds are widely recognized for their therapeutic benefits, such as regulating physiological processes and supporting weight loss [4]. These properties make them suitable candidate agents for obesity treatment. Furthermore, plant-derived extracts exert beneficial responses, such as inhibiting gluconeogenic and lipogenic gene expression, reducing insulin resistance and inflammation, and protecting against oxidative liver damage [5–9]. However, no anti-obesity agent has been identified as the most effective to date.
The leaf extract of Philadelphus schrenkii, an East Asia native species used in traditional medicine [10], exhibits antibacterial [11], antioxidant [12], and anti-inflammatory [13] properties. Such properties are associated with metabolic benefits, including improved insulin sensitivity and reduced adipose tissue inflammation [14]. However, despite these known advantages, whether P. schrenkii can directly influence key mechanisms in adipose tissue to mitigate obesity remains unexplored.
One promising strategy for obesity treatment is the activation of thermogenic adipocytes, which are brown and beige fat cells, to enhance energy utilization [15]. These specialized adipocytes dissipate energy as heat through nonshivering thermogenesis, which is a process primarily mediated by uncoupling protein 1 (UCP1) [16]. This process involves the coordinated expression of proteins participating in thermogenesis and mitochondrial oxidative phosphorylation (OXPHOS) [16]. Thermogenic adipocyte activation has been associated with increased energy expenditure [17], improved glucose homeostasis [18], and lipid metabolism [18], making it a multifaceted strategy against obesity.
In this study, we investigated the anti-obesity properties of P. schrenkii methanol extract (PSE), selected as a candidate from a screening of 105 plant-derived samples, in adipocytes in vitro, as well as normal or obese mouse models in vivo. We examined the molecular changes associated with PSE treatment, focusing on thermogenic markers, to elucidate its possible physiological benefits.
MATERIALS AND METHODS
Plant extracts were obtained from the Natural Product Central Bank at the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The detailed information on the plant samples is presented in Supplementary Table S1. Each plant was dried under the shade for 2 weeks and extracted with methyl alcohol 99.9 % (HPLC grade) as the extract solvent in an enclosed ultrasonic extractor (SDN-900H, SD-Ultrasonic, Seoul, Korea) under the following conditions: 1,500 W, 40 kHz, 120 min ultrasonication, standing per cycle, and 30 cycles. After extraction, the mixture was filtered (Qualitative Filter No. 100, Hyundai Micro, Anseong, Korea) at room temperature and dried under reduced pressure.
3T3-L1 cells were cultured in DMEM with 10% fetal bovine serum (FBS) and penicillin–streptomycin (10 U/mL, 15140163, Thermo Fisher Scientific, Waltham, MA, USA) and maintained in a 5% CO2 incubator at 37℃ until confluent. 3T3-L1 preadipocytes were seeded at a density of 1 × 104 cells/well in 96-well plates to confirm the effects of plant extracts on cell viability. They were treated with 50 μg/mL plant extracts. After 24, 48, and 72 hr of incubation with the treatment, 10 μL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (Amresco, San Francisco, CA, USA) was added to each well, and the cells were incubated at 37℃ for 3 hr. After the supernatant was removed, 0.04 M HCl–isopropanol was added to each well with the same volume as the media to dissolve formazan crystals. The absorbance of each well was measured at 540 nm by using the microplate reader. The optical density of the control cells (untreated) was taken as 100% viability.
For adipocyte differentiation, 3T3-L1 preadipocytes were seeded at a density of 5 × 103 cells/well in six-well plates. After 48 hr of incubation, they were incubated with differentiation induction medium I (DMEM with 10% FBS, penicillin–streptomycin, 0.5 μg/mL insulin [I5500, Sigma-Aldrich, St. Louis, MO, USA], dexamethasone [5 μM, D4902, Sigma-Aldrich], and 3-isobutyl-1-methylxanthine [IBMX, 0.5 mM, I7018, Sigma-Aldrich]) for 72 hr and then in differentiation induction medium II (DMEM with 10% FBS, 10 U/mL penicillin-streptomycin, and 10 μg/mL insulin) for another 72 hr. The plant extracts (50 μg/mL) were added during each medium change throughout the differentiation period.
Afterward, the cells were washed twice with phosphate-buffered saline (PBS) and added with 1 mL of propylene glycol to each well for 2 min. Propylene glycol was removed, and the cells were washed again with PBS. Then, they were stained with 0.5 mL of the working solution of Oil Red O (ORO; O0625, Sigma-Aldrich) for 20 min. They were washed with 85% propylene glycol and added with 1 mL of distilled water to remove excess stain. The stained lipid droplets were visualized using a light microscope (Leica Microsystems, Wetzlar, Germany). Subsequently, the cells were rinsed twice with distilled water, and the remaining ORO dye was extracted using 0.5 mL of isopropanol for 10 min. Absorbance was determined at 500 nm by using a multi-well microplate reader (BioTek, Agilent, Santa Clara, CA, USA). Results were normalized to the optical density of the untreated control cells set as 100%.
For 10T cells, differentiation was induced using differentiation induction medium I containing DMEM, 10% FBS, 10 U/mL penicillin–streptomycin, insulin (10 μg/mL), dexamethasone (1 μM), and IBMX (0.5 mM). After 48 hr of incubation, the medium was replaced with a complete medium supplemented with insulin (10 μg/mL), and this process was repeated twice. For the analysis of 10T cell differentiation, PSE (50 μg/mL) was added during each medium change throughout the differentiation period. For mRNA and protein analysis, PSE was added to the fully differentiated cells for 24 hr.
All mouse experimental procedures were approved by the Institutional Animal Care and Use Committee of Chonnam National University (CNU IACUC-YB-2024-208). Eight-week-old C57BL/6N male mice (Damul Science, Seoul, Korea) were used in this study and given free access to food and water under a 12 hr light/dark cycle at 22℃ with 55% ± 5% relative humidity. For diet-induced obesity, the mice were provided with a high-fat diet (HFD; 60% calorie fat; D12492, Research Diets, New Brunswick, NJ, USA).
PSE was prepared by dissolving the extract in 0.12% DMSO and administered orally via gavage at doses of 15 mg/kg (low concentration, PSE-L) and 30 mg/kg (high concentration, PSE-H) five times per week for 17 weeks. Food intake was manually recorded, and body weight was monitored weekly. At the time of sacrifice, the mice were euthanized by CO₂ inhalation in accordance with institutional ethical guidelines. Wet tissue weights were measured with a balance scale, and tissue samples were either snap-frozen for storage or transferred to embedding cassettes for further analysis.
Before GTT was conducted on the 11th week of chow diet (CD) or 14th week of HFD feeding, the mice were fasted for 16 hr and intraperitoneally injected with glucose (2 g/kg of body weight). Tail vein blood was collected to measure glucose levels at each time point by using an Accu-Check (Performa, Roche, Indianapolis, IN, USA).
Cell lysis and tissue homogenization were performed using radioimmunoprecipitation buffer (150 mM NaCl, 50 mM Tris, pH 8.0, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 1X NP-40) with a protease inhibitor cocktail (11836153001, Sigma-Aldrich). Protein concentrations were quantified using DC Protein Assay Reagents (5000116, Bio-Rad, Hercules, CA, USA), and an equal amount of protein per sample was purified using the acetone precipitation method [19]. The resulting pellet was diluted in SDS sample buffer (60 mM Tris at pH 6.8, 25% glycerol, 0.1% bromophenol blue, 2% SDS, 2.5% 2-mercaptoethanol). Protein lysates were subjected to SDS-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes. The blots were incubated with the following antibodies: fatty acid synthase (FAS; ab22759), UCP1 (ab10983), and total OXPHOS (ab1104413, Abcam, Cambridge, UK); p-PKA substrate (9621) acetyl-coA carboxylase (ACC; 3676), p-HSL (pS660; 4126), and HSP90 (4874, Cell Signaling Technology, Danvers, MA, USA); and p-Perilipin 1 (4856, Vala Sciences, San Diego, CA, USA) as the loading control. The antibodies were prepared and diluted in accordance with the manufacturer’s recommendations. Protein bands were visualized using a luminescent image analyzer (29399481 Amersham ImageQuant 800, Cytiva, Marlborough, MA, USA). Protein expression levels were quantified via densitometry analysis in ImageJ.
Upon sacrifice, the adipose tissues and livers of the mice were dissected, transferred to tissue blocks, fixed in a 10% neutral buffered formalin solution, and embedded in paraffin. Then, 5 µm (interscapular brown adipose tissue or iBAT, liver) or 7 µm (inguinal white adipose tissue or iWAT, epididymal white adipose tissue or eWAT) sections were stained using hematoxylin and eosin. The adipocyte area was quantified using ImageJ.
Mouse serum was examined using an Auto chemistry analyzer (Dotto 2000, MTD Medical System, Como, Italy) to measure the following variables: glucose, alanine transaminase, aspartate aminotransferase, cholesterol, and triglyceride levels.
Total RNA was isolated from frozen adipose tissues by using TRI-Solution (TS200-001, BioScience Technology, Seoul, Korea) in accordance with the manufacturer’s instructions. The cDNA library of the isolated total RNA was generated using Moloney murine leukemia virus reverse transcriptase (28025021, Thermo Fisher Scientific) and oligo dT. Gene expression was analyzed through quantitative PCR by using a QuantStudio 6 real-time PCR system (4485691, Thermo Fisher Scientific). For data normalization, TATA-box binding protein (Tbp) mRNA was used as the reference gene. The primer sequences used in this study are listed in Supplementary Table S2.
All data reported in this study were expressed as the mean ± the S.E.M. and analyzed from individually collected biological samples. One-way ANOVA was performed when repeated measurements were conducted over an extended period. Statistical significance across experimental groups was examined via Student’s t-test. Data were analyzed and visualized using GraphPad Prism (version 9.3.1, GraphPad Software, San Diego, CA, USA).
RESULTS
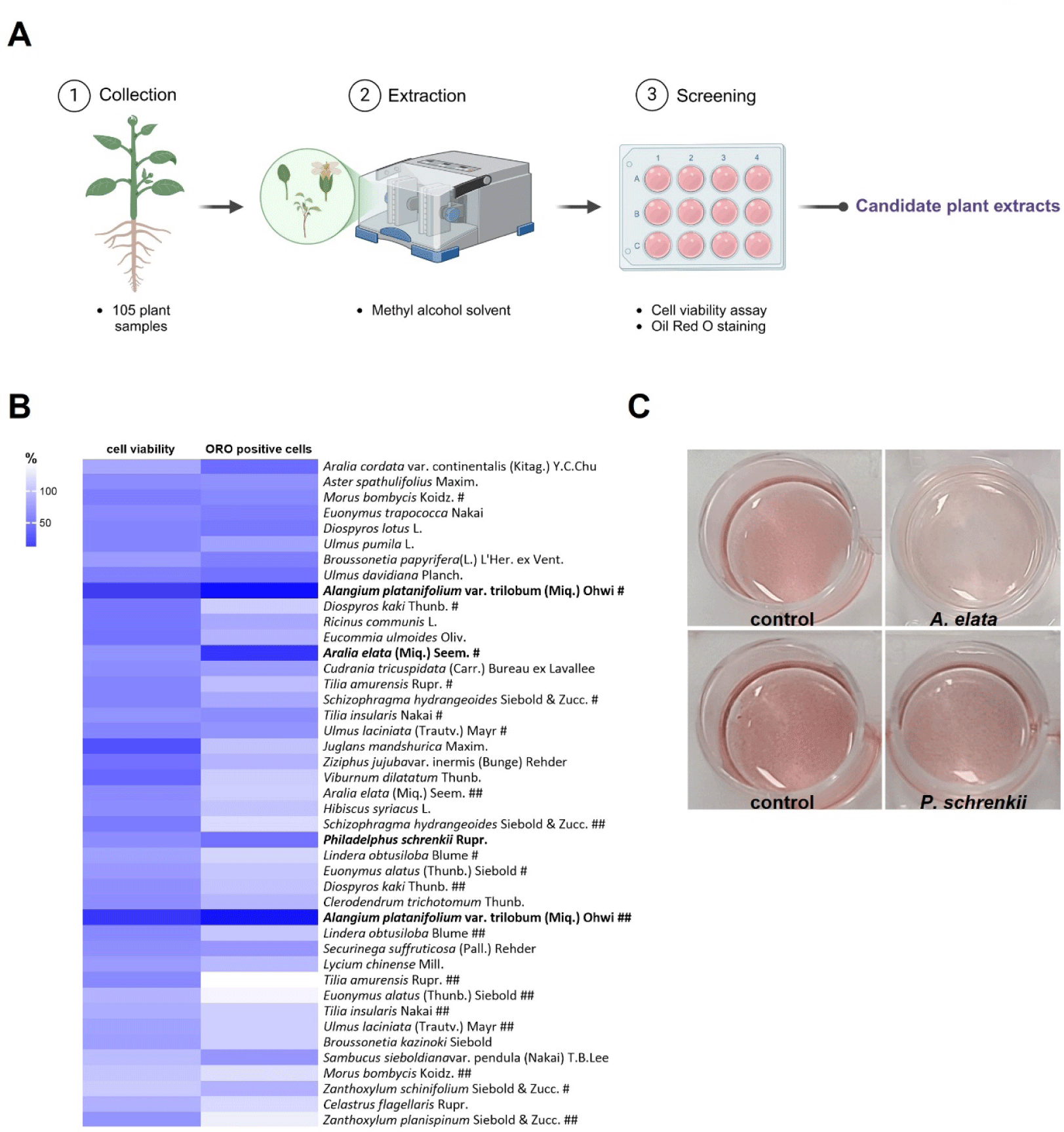
A total of 105 plant samples obtained from the Natural Product Central Bank at the Korea Research Institute of Bioscience and Biotechnology were extracted in methanol and screened for their cytotoxic and anti-adipogenic effects on 3T3-L1 cells (Fig. 1A). The cells were treated with the extracts derived from various plant parts and species to assess cytotoxicity (Fig. 1B, Supplementary Fig. S1A). The cell viability assay indicated that the cells survived in most leaf extracts, while some, particularly those from Alangium platanifolium (A. platanifolium) collected at two different periods, significantly reduced cell viability (Fig. 1B, Supplementary Fig. S1A), indicating their cytotoxic effects. ORO staining analysis revealed that lipid accumulation, a hallmark of fully differentiated adipocytes, was markedly reduced in 3T3-L1 cells treated with A. platanifolium, Aralia elata, or P. schrenkii extract (Fig. 1B and C, Supplementary Fig. S1B). On the basis of the cytotoxicity and ORO staining results, A. platanifolium extract was excluded, and only P. schrenkii leaf extract was selected as a candidate for further investigation into its anti-obesity potential. Although A. elataleaf extracts exhibited low cytotoxicity and markedly reduced lipid droplets (Fig. 1B and C), this species was also excluded from subsequent analyses because of a previous publication describing its anti-obesity effects [20].
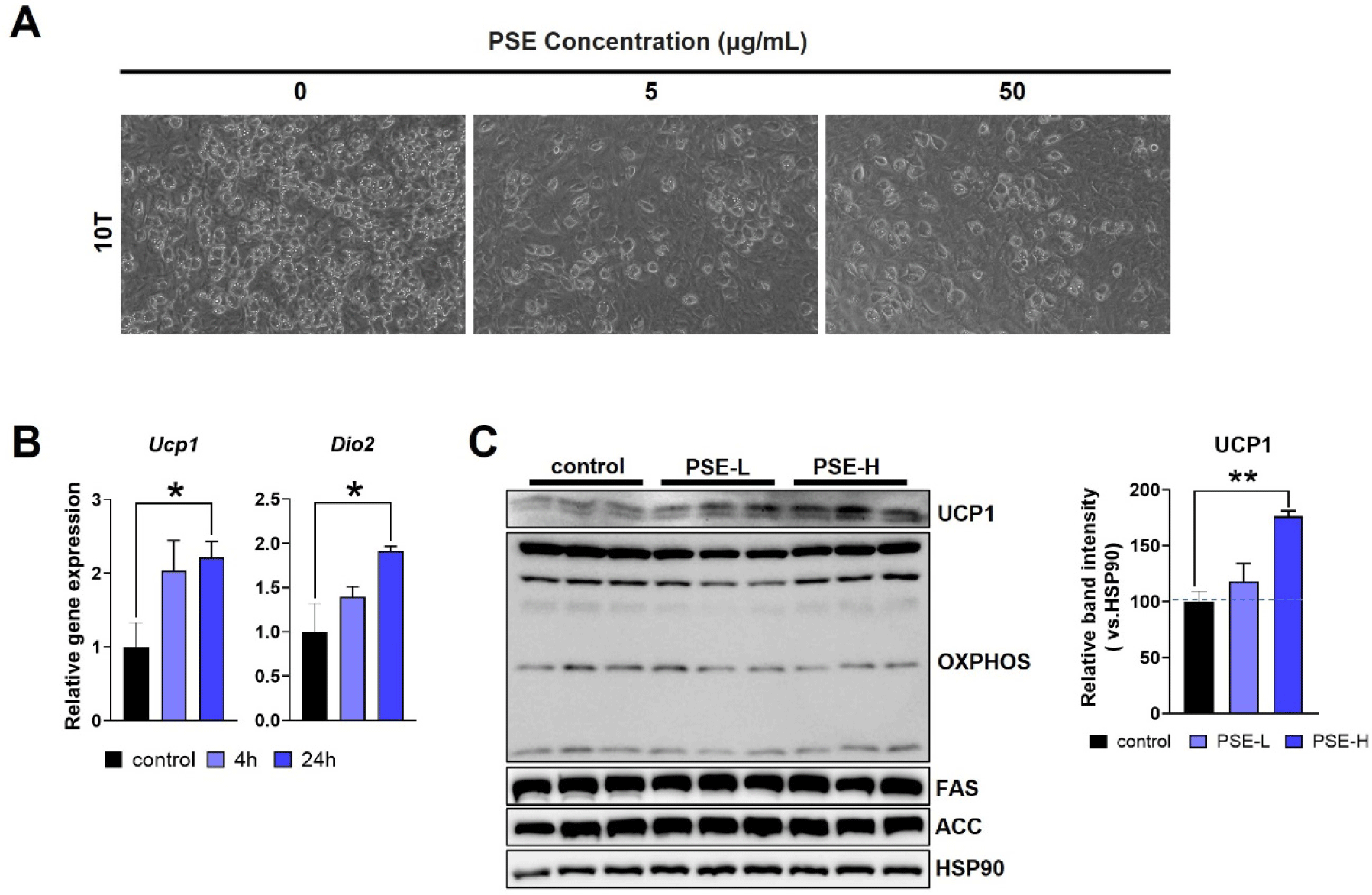
To further confirm the anti-adipogenic effects of PSE in vitro, we treated C3H10T1/2 (10T) cells with 5 µg/mL (PSE-L) or 50 µg/mL (PSE-H) of PSE during differentiation. The PSE treatment reduced the lipid droplet formation in 10T cells, suggesting that adipogenesis was inhibited (Fig. 2A). The PSE-H treatment in fully differentiated 10T cells for 24 hr increased the expression of Ucp1 and Dio2, genes involved in thermogenesis (Fig. 2B). The UCP1 protein levels were also upregulated by PSE-H, while no changes were observed in the lipogenic proteins FAS and ACC or in OXPHOS proteins (Fig. 2C). These results suggest that PSE exerts anti-adipogenic and thermogenic effects on cultured adipocytes.
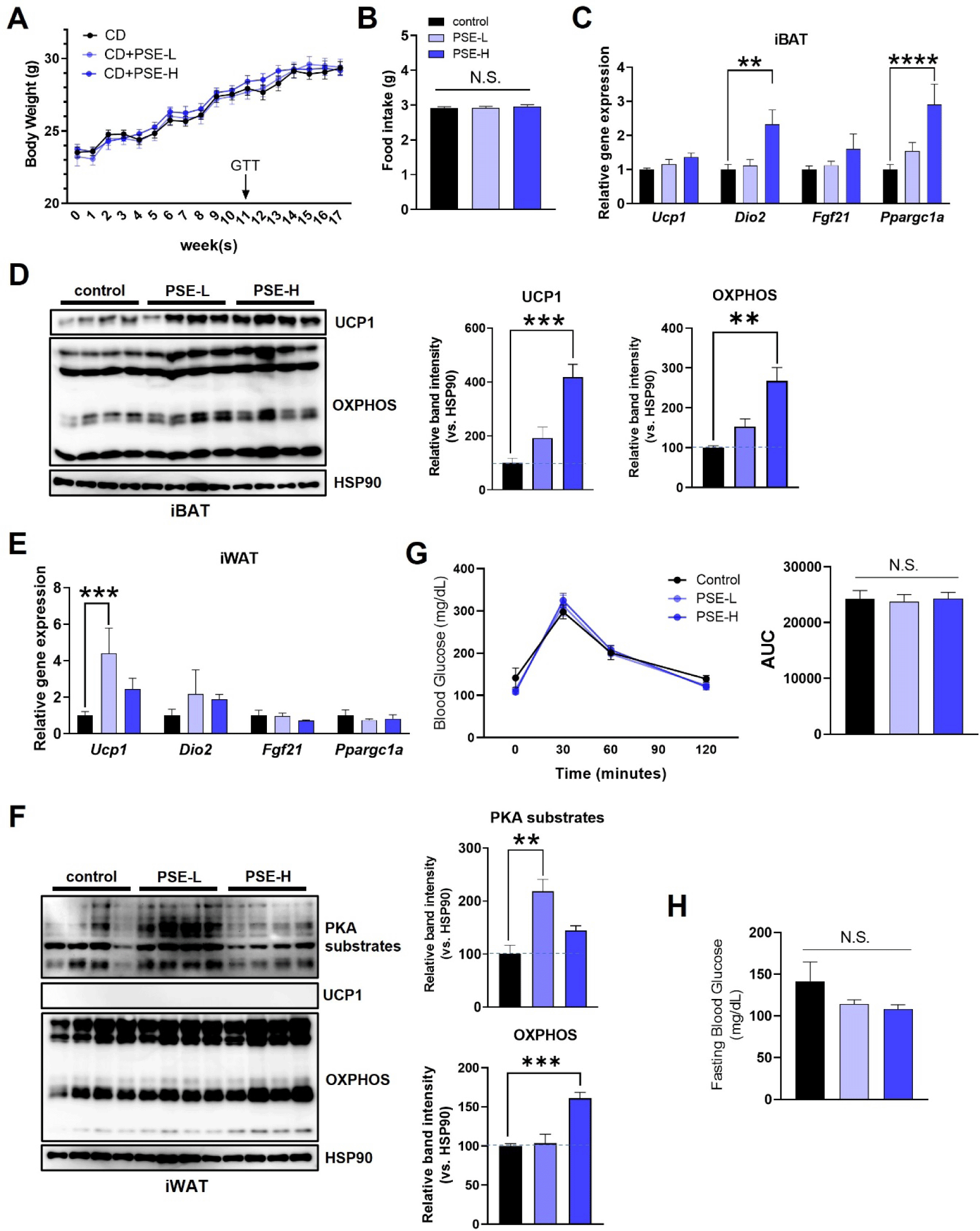
To reveal the effects of PSE treatment on adipocytes in vivo, we treated the CD-fed mice with 15 mg/kg (PSE-L) or 30 mg/kg (PSE-H) PSE via oral gavage for 17 weeks. Both treatment doses of PSE did not cause significant differences in body weight, food intake, and tissue weights compared with those of the control mice (Fig. 3A and B, Supplementary Figs. S2A and S2B). The expression levels of thermogenic genes, such as Dio2 and Ppargc1a mRNA, in the iBAT, the primary site of non-shivering thermogenesis, significantly increased (Fig. 3C). This increase was accompanied by the upregulation of UCP1 and OXPHOS protein levels, which increased by approximately 400% and 270% in the high-dose group compared with those in the control group, respectively (Fig. 3C and D). In iWAT, another site of thermogenic activation through the formation of beige adipocytes, PSE-L increased the Ucp1 expression, although UCP1 levels were undetectable (Fig. 3E and F). The activity of protein kinase A (PKA), a key factor in the classical thermogenic pathway determined by PKA substrate phosphorylation, and the protein level of OXPHOS increased in PSE-administered mice (Fig. 3F). In eWAT, the levels of proteins involved in lipolysis (p-perilipin and p-HSL) or lipogenesis (ACC and FAS) did not significantly change (Supplementary Fig. S2C). Despite the enhanced thermogenic factors, no histological differences, glucose tolerance, or fasting blood glucose changes were observed between the groups (Fig. 3G and H, Supplementary Fig. S2D). These results suggest that PSE upregulates factors related to thermogenesis in mice fed a CD.
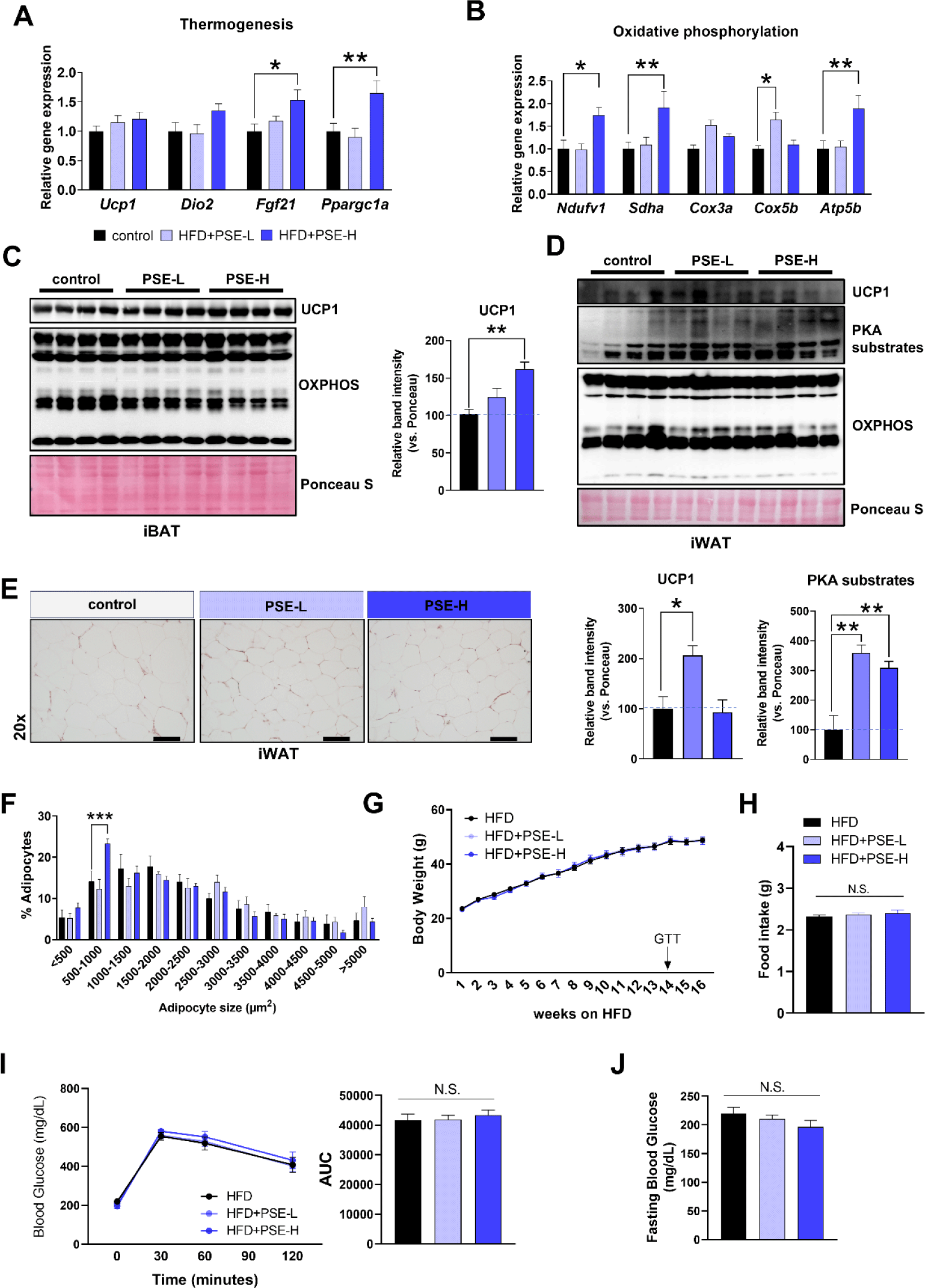
Based on the anti-adipogenic and thermogenic effects of PSE, we investigated whether PSE treatment could benefit the diet-induced obese mice. Mice were fed with HFD for 16 weeks with or without PSE treatment. In the PSE-treated group, thermogenic- and OXPHOS-related genes, including Fgf21, Ppargc1a, Ndufv1, Sdha, Cox5b, and Atp5b, were upregulated in iBAT compared with those in the HFD-only group (Fig. 4A and B). UCP1 levels were significantly upregulated in the high-dose PSE-treated group (Fig. 4C). In iWAT, low-dose PSE increased UCP1 levels, while both doses increased PKA activities (Fig. 4D). The number of smaller adipocytes in iWAT significantly increased in the high-dose group, indicating that adiposity was reduced (Fig. 4E and F). Despite these improvements, body weight, fat and liver tissue weights, food intake, glucose tolerance, fasting blood glucose, serum triglyceride, or serum cholesterol levels did not significantly differ (Fig. 4G, H, I, and J, Supplementary Fig. S3A, S3B, and S3C). The histological analyses of iBAT, eWAT, and liver also revealed no notable differences between the groups (Supplementary Fig. S3D). Furthermore, there were no differences in the levels of liver damage markers such as ALT and AST between groups (Supplementary Fig. S3C). Overall, the upregulation of thermogenic UCP1 and reduction in iWAT adiposity suggest that PSE may be a potential candidate for targeting adipose tissue function in obesity management.
DISCUSSION
The development of effective obesity treatments remains a significant challenge due to the limitations of existing pharmacological and surgical options [1, 2]. For instance, the use of GLP-1 analogs, such as liraglutide and semaglutide, as weight-loss drugs is controversial for their associated increased risk of pancreatitis, gastrointestinal adverse effects, and psychiatric problems [21–23]. Bariatric surgery, which is an effective long-term treatment for severe obesity, involves invasive procedures that pose risks such as bleeding and infections [2]. Therefore, in the present study, we sought to explore a plant-based alternative with anti-obesity potential. Leaf extracts from various plant species are known to harbor bioactive compounds with therapeutic effects against obesity [3, 24–26]. Most of them, including A. elataleaf extracts [20], have been reported to target lipid metabolism or inhibit pancreatic lipase activity [3, 4]. This study highlights the novel mechanism of action of PSE, derived from the methanolic extraction of P. schrenkii leaves, through its ability to enhance the expression of thermogenesis-related proteins. The safety of PSE was also confirmed through cytotoxicity assays and unaltered ALT and AST levels in mice, proving its suitability as a supportive anti-obesity agent.
Our results demonstrated that the oral administration of PSE significantly increased the expression of thermogenic UCP1 in 10T cells and adipose tissues of PSE-treated mice. Notably, PKA substrate phosphorylation was enhanced in iWAT, accompanied by an increase in smaller adipocytes, indicating a potential enhancement of the lipolytic pathway. Adipose thermogenesis is primarily attributed to the abundant mitochondria and the expression of UCP1 in brown and beige adipocytes [15]. Therefore, compounds that activate this pathway may cause therapeutic effects by promoting fat utilization. Despite the observed changes, PSE administration did not significantly affect body weight or serum parameters such as glucose, triglyceride, and cholesterol in HFD-fed mice. This outcome is consistent with growing evidence showing that UCP1 induction alone is inadequate to increase thermogenic output, especially if not augmented by activated electron transport chain activity [27]. In addition, UCP1-independent thermogenic pathways also contribute substantially to brown and beige fat thermogenesis [28–30], and without activation of these parallel processes, upregulation of UCP1 is unlikely to translate into measurable physiological benefits. These findings suggested that the effects of PSE were insufficient to induce weight loss, although it enhanced the adipose tissue function, as evidenced by the increased thermogenic UCP1 expression, PKA signaling activation, and reduction in iWAT adiposity. Future studies should investigate combinatorial approaches or extended treatment durations of PSE to enhance its efficacy. It should also be noted that the composition of plant-derived compounds is influenced by the extraction method, with solvent polarity determining the yield of phytochemicals and antioxidants that are most efficiently recovered [31]. Different extraction approaches can yield extracts with distinct biological actions. Methanol extraction, for example, typically enriches semi-polar phytochemicals such as flavonoids and phenolic acids, whereas water extraction favors more polar constituents like polysaccharides and glycosides [32]. Because PSE is a complex extract, isolating and identifying its active components will also be important for elucidating the mechanisms underlying its thermogenic effects. This will allow for the development of possibly more potent therapeutic agents.
Nonetheless, given its safety profile and ability to regulate thermogenic UCP1 expression in adipose tissues, PSE holds promise as a supportive component of plant-based obesity therapies. Although its standalone effects may be modest, PSE could complement existing treatments by enhancing thermogenic protein expression and reducing adiposity.







