INTRODUCTION
The incidence of esophageal perforation (EP) has increased during the past 40 years because of greater use of endoscopic procedures. In spite of recent advances in surgery and medical treatment, EP is a life-threatening condition which a mortality is close to 20% [1]. We report a case of EP due to the insertion of a calibration tube during sleeve gastrectomy even though there were no detectable problems in the insertion or adjustment of calibration tube due to mucosal inflammation.
CASE REPORT
A consent was obtained for the publication of this report, which was approved by the Hospital Institutional Review Board. A 36-year-old, 156 cm, 85 kg, obese (body mass index, 34.5 kg/m2) woman was scheduled for sleeve gastrectomy with laparoscopy. She had controlled diabetes mellitus, hypertension, a mild fatty liver, and gastroesophageal reflux disease (GERD), observed on pre-surgical endoscopy. Anesthesia was induced with 150 mg propofol, 60 mg rocuronium, and 0.1 µg/kg/min remifentanil. The patient had a Cormack–Lehane grade of 2. Intubation was performed without any difficulty using a 7.0-mm endotracheal tube (MallinckrodtTM, Covidien, Dublin, Irland). Intra-abdominal pressure during surgery was maintained at 14 mmHg or lower, and a calibration tube was inserted through the oropharynx after starting the surgery. The calibration tube was obtained from the LAP-BAND Adjustable Gastric Banding System, access port II™, with an external diameter of 13 mm (LAP-BNND®, Allergan, Irvine, CA, USA). Calibration tube insertion was attempted with laryngoscopic assistance after applying sufficient lubrication, and up to 30 cm of the tube was inserted into the esophagus without resistance. At 30 cm, a slight resistance was felt. After backing up about 5 cm, insertion was attempted again. The tube was inserted up to 60 cm, passing through the gastroesophageal (GE) junction without resistance. Aspiration of gastric juice was performed through the tube with no blood observed. The location of the tube in the stomach was confirmed with the laparoscope screen. Distal ballooning was intentionally not performed when inserting the calibration tube, and it was used only for confirming the location, checking leakage, and preventing stenosis after surgery. The calibration tube was moved 3 times inside the stomach during the surgery. The surgery was completed in 4 hours and 30 minutes without complications. After transfer to the recovery room, the patient regained consciousness, and the blood pressure was maintained at 130/70 mmHg. However, the patient complained of mild dyspnea and chills, and had a heart rate of 130–140 beats/min, respiratory rate of 36 beats/min, oxygen saturation (SpO2) of 92%, and body temperature of 38°C. The oxygen flow rate through the mask was increased to 10 L/min, and a chest radiography was performed. Arterial blood gas analysis was performed, and showed a pH of 7.23, PCO2 of 59 mmHg, PO2 of 60 mmHg, HCO3− of 24 mmol/L, and SpO2 of 85%; both lung fields showed a diffuse haziness on chest radiography. After 2 hours in the recovery room, the patient had a blood pressure of 130/80 mmHg, heart rate of 124 beats/min, SpO2 of 99%, body temperature of 38℃, pH of 7.34, PCO2 of 49 mmHg, PO2 of 155 mmHg, HCO3− of 26 mmol/L, and SpO2 of 99%. The patient’s dyspnea improved, and she was therefore moved to the general ward.
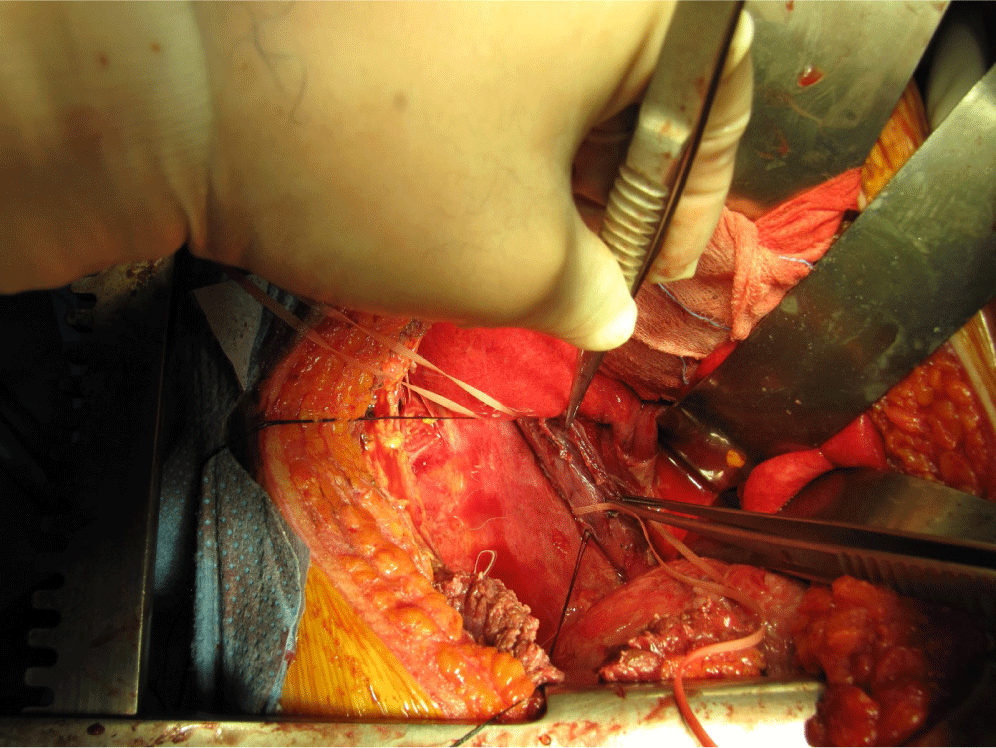
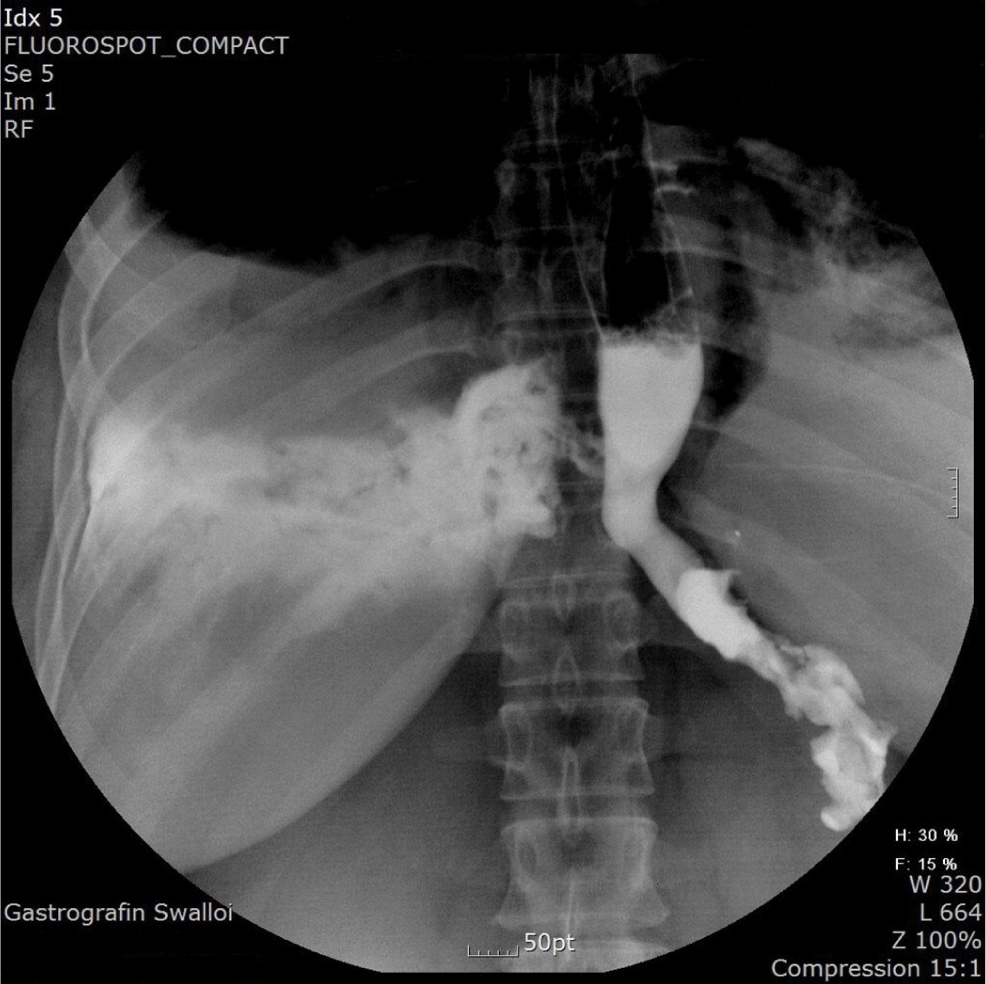
Approximately 4 hours after moving to the general ward, the patient again complained of chest discomfort, dyspnea, chills, and a continuous high fever. The patient showed leukocytosis with a white blood cell count of 22,400/mm3. Chest radiography and a Gastrografin swallowing test were performed. The chest radiograph showed pneumonic consolidation with pleural effusion in the right lung. The Gastrografin swallowing test results showed an EP with dye leaking to the right pleura (Fig. 1). Under a diagnosis of EP with acute mediastinitis, a thoracotomy was performed. A full thickness tear of around 5 cm was observed extending from approximately 2 cm above the GE junction to below the azygos vein (Fig. 2). The edge of the torn tissue was not necrotic, and there was no sign of inflammation. Surgery was performed to repair the tissue and add a reinforcement pleural flap, and catheter drainage was performed to treat the right pleural effusion. A barium study performed 1 week later showed no esophageal leakage; however, the right pleural effusion persisted, requiring catheter reinsertion for drainage. Twenty days after the surgery, the patient improved and was discharged in a good condition.
DISCUSSION
Causes of EP can be largely divided into 4 types: trauma, foreign body ingestion, spontaneous, and iatrogenic. Causes from trauma can be gunshots, stab wounds, and cervical spine fractures. Cases owing to blunt trauma are very rare (0.001%), while those from penetrating injury account for approximately 6% of EP cases. Most cases of trauma occur in the cervical esophagus, and its severity is related to the tracheal region.
Cervical EPs from foreign bodies account for approximately 80% of all cases, mostly in the cricopharyngeal muscle. It usually occurs in areas that become narrower anatomically, such as the left main stem bronchus, aortic arch, and lower esophageal sphincter [2].
A spontaneous EP is a barotraumatic rupture of the lower third of the esophagus caused by a sudden increase in the intraesophageal pressure without relaxation of the superior sphincter of the esophagus. It is called Boerhaave syndrome when it is caused by vomiting or retching, and its incidence is approximately 15%. Approximately 80% of cases occur in the left posterolateral wall border of the lower third of the thoracic esophagus, 2–3 cm above the GE junction, and an approximately 3–6 cm long perforation occurs [2, 3].
Finally, iatrogenic causes of EP are surgery and orogastric instrument insertion, such as an endoscopic intervention and nasogastric tube insertion. Cases caused by surgery occur when operating on the esophagus or surrounding organs. Cervical spine fracture surgery occurs mostly after osteosynthesis, and the incidence is approximately 3.4%. Thoracic EPs also occur after bronchial artery embolization or lung resection and usually occur in the lower third of the esophagus when performing anti-reflux surgery for GERD or myotomy in achalasia [2, 4, 5].
In the current case, foreign body ingestion and trauma were ruled out as causes of EP. The perforated area was in the thoracic distal esophagus, where spontaneous perforation frequently occurs, but there were no abnormalities other than GERD detected during the pre-surgical endoscopy. There were no causative events before or during induction of anesthesia, and there is low probability of vomiting or retching because the muscles are already relaxed in the anesthetic state. In addition, spontaneous EPs are usually small, transmural tears, but the patient had a full thickness tear of approximately 5 cm; therefore, there is low probability that the cause of this EP was spontaneous perforation such as in Boerhaave syndrome.
Thus, the cause of this case was likely iatrogenic, the most common cause of EPs. Iatrogenic cases, such as spontaneous EPs, are usually in the narrowest area of the thoracic esophagus, as in this case [2]. The majority of iatrogenic EPs reported to date have been due to intragastric balloon extraction or calibration tube insertion [6]. EP caused by a calibration tube usually occurs in the thoracic distal esophagus for unspecified reasons [7, 8].
Iannelli et al. reported a perforation caused by accidental distal balloon inflation of the calibration tube inside the intrathoracic esophagus and not the stomach, and reported that it was caused by a sudden increase in intraesophageal pressure similar to that in Boerhaave syndrome [9]. The standard method for inserting a calibration tube in laparoscopic bariatric surgery is to sufficiently insert the calibration tube and then inject 15–30 mL of saline into the distal balloon. After ballooning, the tube is pulled up to the GE junction to perform the banding procedure. However, in the present case, the surgeon had previously experienced an EP complication during banding surgery; therefore, distal ballooning was intentionally not performed when inserting the calibration tube, and it was used only for confirming the location, checking leakage, and preventing stenosis after surgery. For this reason, the present EP case was likely not caused by accidental distal ballooning of the calibration tube. The patient showed signs of GERD on the pre-surgical endoscopy. And the tear in this case may have occurred from the forward and backward movement at insertion, and the repeated location adjustments of the calibration tube when the mucosal area was already weakened from GERD.
When performing laparoscopic gastrectomy, some surgeons refrain from using a calibration tube, but not in most cases. In principle, refraining from using a calibration tube during surgery can prevent EP. However, when it is used, it should be carefully performed by an anesthesiologist experienced in this procedure. In addition, the patient should be closely monitored during and after surgery, and when there are symptoms, prompt diagnosis and treatment of the patient should be performed.
An EP can occur at any time, as in the present case where an EP occurred even though there were no problems in the insertion or adjustment of the calibration tube. Subsequent complications accompanying EP can be fatal. Even slight mucosal inflammation can heighten the risk of EP, as in the current case. EP caused by a calibration tube during gastrectomy is rare and there has been no discussion in the literature regarding potential risk factors, such as mucosal inflammation. Our case report could be important for showing that the risk of EP can increase in the presence of mucosal inflammation.