INTRODUCTION
Human dermal fibroblasts (HDFs) are fundamental to the structure and function of the skin, particularly the dermis, where they are responsible for producing and maintaining extracellular matrix (ECM) components such as collagen, elastin, and fibronectin [1]. These cells also secrete cytokines and growth factors that support epidermal integrity and coordinate wound healing. In response to injury or skin aging, fibroblasts mediate re-epithelialization, contract the wound bed, and promote angiogenesis. However, under pathophysiological conditions such as oxidative stress, nutrient deprivation, or mechanical injury, fibroblasts undergo apoptosis, which impairs dermal remodeling and delays tissue repair [2, 3]. In aging skin, decreased fibroblast viability contributes to ECM degradation, thinning of the dermis, and reduced regenerative capacity [4].
Given the central role of fibroblasts in skin regeneration, strategies that enhance fibroblast survival under stress conditions are of significant therapeutic interest. Recent attention has turned to the use of mesenchymal stem cells, particularly adipose-derived stem cells (ADSCs), due to their potent paracrine effects and tissue repair properties [5]. ADSCs are easily accessible, exhibit stable expansion in vitro, and are capable of secreting a broad repertoire of trophic factors, including cytokines, chemokines, growth factors, and extracellular vesicles [6]. These secretions have been shown to promote the proliferation and migration of keratinocytes, modulate inflammation, and enhance the survival of fibroblasts in injured or aging tissue microenvironments [7].
Among the bioactive molecules secreted by ADSCs, hepatocyte growth factor (HGF) has emerged as a particularly important mediator of cellular repair and survival. HGF is a pleiotropic growth factor that acts through its cognate receptor, c-Met, a receptor tyrosine kinase widely expressed in epithelial and mesenchymal cells, including fibroblasts [8]. Upon binding to c-Met, HGF activates multiple downstream pathways such as PI3K/AKT, MAPK/ERK, and STAT3, which regulate diverse cellular processes including proliferation, motility, differentiation, and anti-apoptotic signaling [9]. Among these, the PI3K/AKT pathway is especially well-characterized for its role in promoting cell survival and suppressing apoptosis under various forms of cellular stress [10].
Although the cytoprotective effects of HGF have been demonstrated in several tissues, including the liver, lung, and nervous system, its specific role in the context of ADSC-mediated dermal fibroblast protection remains to be fully elucidated. In particular, whether ADSC-secreted HGF is sufficient to rescue HDFs from apoptosis under low-nutrient or stress-inducing conditions, and whether this occurs through the canonical c-Met–PI3K/AKT axis, has not been clearly established.
In this study, we investigated the anti-apoptotic role of ADSCs on HDFs under nutrient-limited conditions. We employed a Transwell co-culture system to assess paracrine interactions and focused on HGF as a key mediator. By analyzing apoptosis markers, receptor expression, and downstream signaling activity, we aimed to determine whether HGF secreted by ADSCs engages c-Met–AKT signaling to confer cytoprotection. Our findings offer novel insights into the molecular mechanisms underlying stem cell–fibroblast communication and support the therapeutic utility of ADSCs in skin regeneration.
MATERIALS AND METHODS
HDFs (PromoCell, Heidelberg, Germany) and ADSCs (PromoCell) were cultured following the supplier’s instructions. HDFs were maintained in Fibroblast Growth Medium 2 (PromoCell), and ADSCs were cultured in Mesenchymal Stem Cell Growth Medium 2 (PromoCell) at 37°C in a humidified incubator with 5% CO2. HDFs were maintained in serum-reduced DMEM (0.1% FBS) to simulate stress conditions. The initial seeding density (5 × 104 cells/well in a 6-well plate) was determined based on preliminary optimization to ensure optimal cell confluency and reproducibility under low-serum stress conditions. All cells used in this study were from passages 3–5 and tested negative for mycoplasma contamination.
Transwell co-culture system (0.4 μm pore size, Corning, NY, USA) was used to investigate the paracrine effect of ADSCs on HDFs. HDFs were seeded in 6-well plates at a density of 2 × 105 cells/well, and ADSCs were seeded in the upper chamber (Transwell insert) at 1 × 105 cells/insert. After overnight attachment, the culture medium was replaced with serum-reduced DMEM (0.1% FBS) to induce apoptotic stress. The co-culture was maintained for 24, 48, or 72 hr.
To assess the functional role of soluble factors, specific neutralizing antibodies were added to the upper chamber containing ADSCs. HGF-neutralizing antibody and c-Met-neutralizing antibody were obtained from WINDBIO (Seoul, Korea). All antibodies were used at a final concentration of 2 μg/mL. In inhibitor studies, HDFs were treated with LY294002 (10 μM), PD98059 (20 μM), or SB203580 (10 μM; all from Cell Signaling Technology, Danvers, MA, USA).
Apoptosis was assessed using the APOPercentage™ apoptosis assay kit (Biocolor, Carrickfergus, UK), following the manufacturer’s protocol. The assay specifically stains cells undergoing the membrane ‘flip-flop’ event, indicative of apoptotic but not necrotic death. Bright-field images of the pink-stained apoptotic cells were acquired from five randomly selected fields per well at × 100 magnification. Apoptotic activity was quantified by calculating the total number of red-stained pixels using Adobe Photoshop’s histogram function with a fixed color threshold. The average pixel count per field was calculated from three biological replicates.
Conditioned media from ADSCs were collected at 24, 48, and 72 hr for growth factor quantification. Human enzyme-linked immunosorbent assay (ELISA) kits for HGF, insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), and vascular endothelial growth factor (VEGF; R&D Systems, Minneapolis, MN, USA) were used. Each sample was tested in duplicate and normalized to total cell number. Values were expressed as pg/mL.
Surface expression of c-Met in HDFs was measured by flow cytometry using APC-conjugated anti-human c-Met antibody (Abcam, Cambridge, UK). Data were acquired using a FACSCalibur cytometer (BD Biosciences, San Jose, CA, USA). For gene expression analysis, total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). PCR amplification was performed for c-Met and GAPDH, and the resulting products were separated by agarose gel electrophoresis and stained with SYBR Safe DNA Gel Stain (Thermo Fisher Scientific, Waltham, MA, USA). Bands were visualized under UV illumination using a gel documentation system.
Cells were lysed with RIPA buffer containing protease and phosphatase inhibitors. Equal amounts of protein (30 µg) were separated on SDS-PAGE and transferred to PVDF membranes (Millipore, Burlington, MA, USA). Membranes were blocked with 5% Skim milk and incubated with primary antibodies (phospho-AKT, phospho-ERK1/2, phospho-p38, cleaved caspase-3, Bax, Bcl-2, c-Met, and β-actin; Cell Signaling Technology). Detection was performed using HRP-conjugated secondary antibodies and chemiluminescence substrate (Thermo Fisher Scientific).
All experiments were performed in technical triplicate using the same lot of cells. Data are expressed as mean ± S.D. Statistical differences were analyzed using one-way ANOVA followed by Tukey’s post hoc test (GraphPad Prism 8.0, GraphPad Software, San Diego, CA, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
To examine the cytoprotective potential of ADSCs on dermal fibroblasts under stress, apoptosis was induced in HDFs by culturing them under low-serum (0.1% FBS) conditions, which are known to trigger mitochondrial dysfunction and caspase cascade activation in fibroblasts [11]. APOPercentage™ staining revealed significantly higher levels of pink-stained apoptotic cells under serum starvation compared to the 10% FBS control, indicative of enhanced phosphatidylserine externalization and apoptotic membrane flipping. However, the presence of ADSCs in a non-contact Transwell system significantly reduced dye uptake in HDFs (Fig. 1A). Quantitative pixel analysis confirmed a statistically significant reduction in apoptotic staining in the co-culture group (p<0.05), suggesting that soluble factors released by ADSCs confer anti-apoptotic protection.

To further validate this observation, we assessed the expression of key apoptosis-related proteins. Western blot analysis showed that co-culture with ADSCs for 24 and 48 hr resulted in a time-dependent downregulation of cleaved caspase-3 and the pro-apoptotic protein Bax, alongside an increase in the anti-apoptotic protein Bcl-2 (Fig. 1B). These molecular signatures indicate a shift toward survival signaling, consistent with previous reports showing ADSCs can modulate the intrinsic apoptotic pathway through secretome-mediated effects [12].
To identify the specific paracrine mediators underlying the observed anti-apoptotic effect, we quantified levels of several growth factors known to be secreted by ADSCs and implicated in tissue regeneration: HGF, IGF-1, FGF-2, and VEGF. ELISA analysis of ADSC-conditioned media revealed a progressive increase in all factors over time, with HGF exhibiting the highest and most robust fold-change by 72 hr compared to the 24-hr control (Fig. 2A). These findings are consistent with the literature showing that HGF is among the most abundantly secreted cytokines by ADSCs and functions as a potent anti-apoptotic agent in various cell types including epithelial and stromal cells [13, 14].
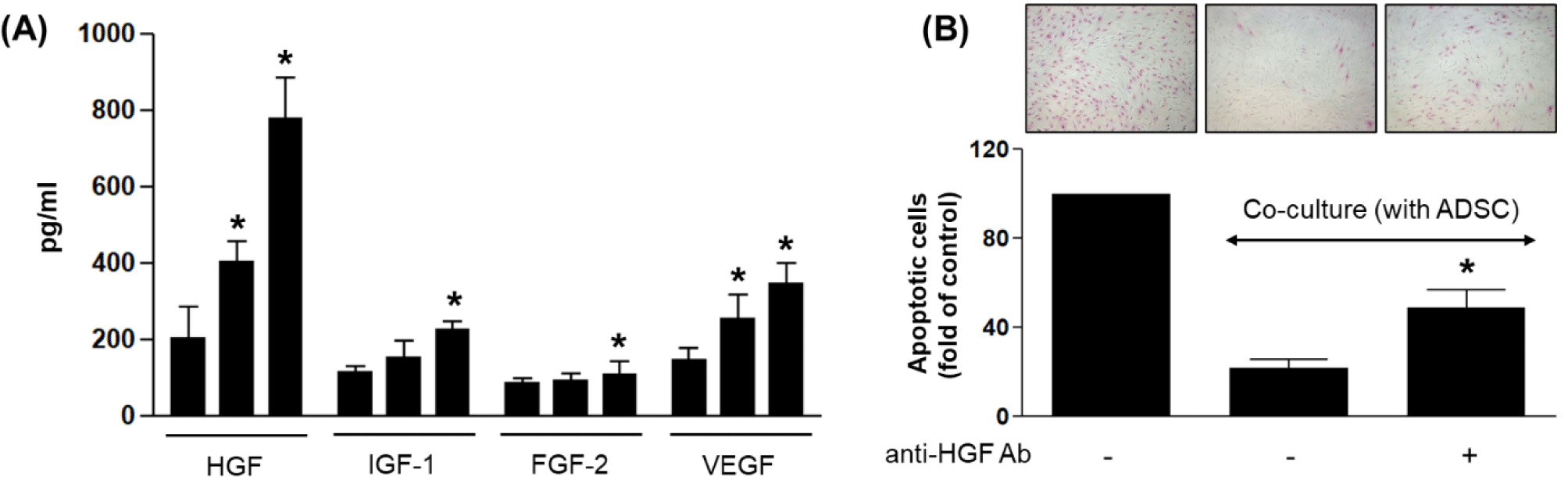
To directly evaluate the role of HGF in mediating the anti-apoptotic response, we treated co-cultures with an HGF-neutralizing antibody. The suppression of apoptosis observed in the ADSC co-culture was significantly reversed upon HGF blockade, as demonstrated by a marked increase in APOPercentage™ staining compared to untreated co-culture controls (p<0.05; Fig. 2B). This indicates that HGF plays a central role in the paracrine-mediated cytoprotection conferred by ADSCs.
To determine whether HDFs are competent to respond to HGF, we examined the expression of its cognate receptor, c-Met. Flow cytometry revealed prominent surface expression of c-Met in HDFs compared to isotype control (Fig. 3A, left), indicating the presence of signaling-competent receptor. At the transcript and protein levels, conventional reverse transcription polymerase chain reaction (RT-PCR) and Western blot analysis confirmed c-Met expression in HDFs (Fig. 3A, right). This expression profile aligns with prior findings demonstrating that dermal fibroblasts constitutively express c-Met and can activate downstream signaling upon HGF stimulation [15].
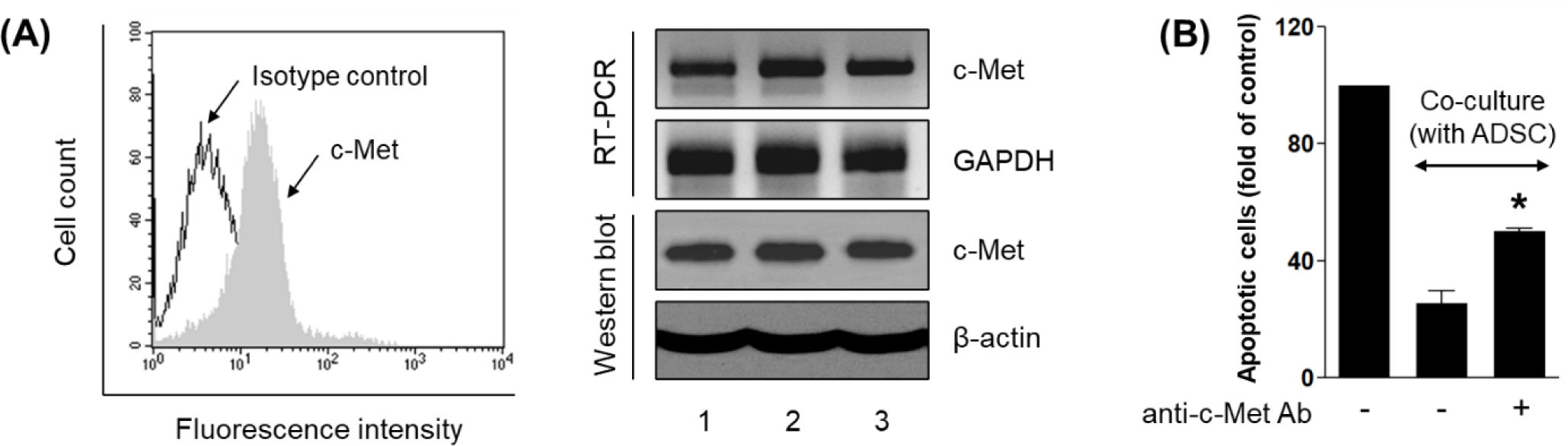
To test whether c-Met is required for HGF-mediated cytoprotection, a c-Met-neutralizing antibody was applied during co-culture. Blocking c-Met effectively abolished the anti-apoptotic effect of ADSCs, as reflected by a significant increase in pink-stained apoptotic cells relative to co-culture controls (p<0.05; Fig. 3B). This finding supports a model in which ADSC-derived HGF interacts with c-Met on HDFs to mediate survival signaling.
To investigate the intracellular signaling pathways downstream of HGF/c-Met activation in HDFs, we analyzed phosphorylation status of AKT, ERK1/2, and p38 MAPK by Western blot. Among these, only phospho-AKT levels were increased in HDFs co-cultured with ADSCs, whereas phospho-ERK1/2 and phospho-p38 remained unchanged (Fig. 4A). These results indicate that PI3K/AKT, but not ERK or p38 MAPK, is selectively activated in response to ADSC-derived signals.
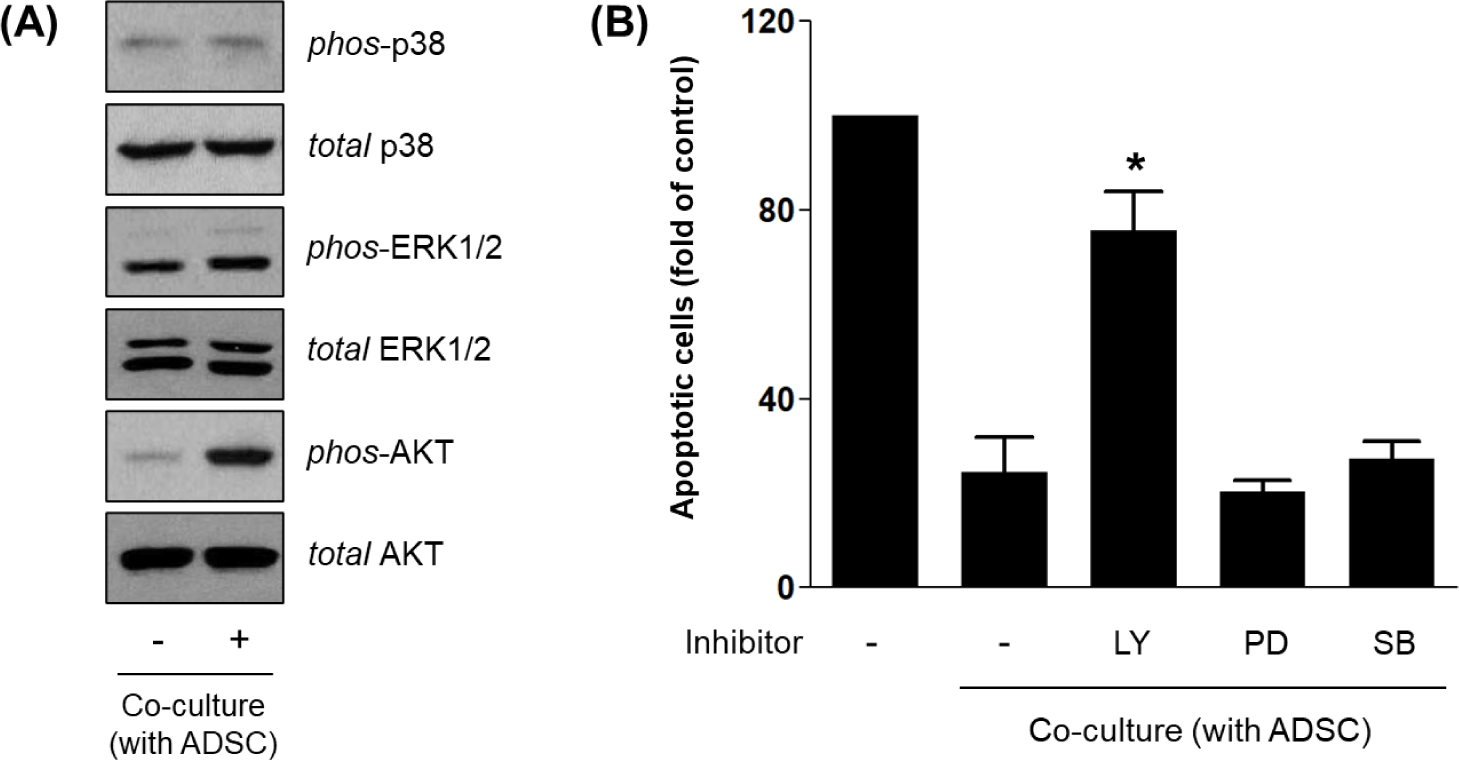
To confirm functional involvement of these pathways, HDFs were treated with specific inhibitors during co-culture. Inhibition of PI3K/AKT signaling by LY294002 significantly reversed the anti-apoptotic effect of ADSCs, restoring apoptotic staining to levels comparable to serum-starved controls. In contrast, inhibition of ERK (PD98059) or p38 (SB203580) had no significant effect on apoptosis (Fig. 4B). Together, these results indicate that ADSC-derived HGF promotes HDF survival primarily through activation of the c-Met–PI3K/AKT axis, consistent with known anti-apoptotic mechanisms in fibroblasts and epithelial cells [16–18].
DISCUSSION
This study demonstrates that ADSCs confer significant anti-apoptotic effects on HDFs under growth factor-limited conditions, primarily through the secretion of HGF and the subsequent activation of the c-Met–PI3K/AKT signaling axis. These findings build on accumulating evidence that ADSCs act as potent paracrine modulators of tissue homeostasis by secreting survival-promoting cytokines and growth factors [19–21].
Serum deprivation is a well-established in vitro model to induce apoptosis in fibroblasts by mimicking physiological conditions of tissue stress, such as ischemia or chronic inflammation [22, 23]. Under these conditions, fibroblasts exhibit mitochondrial dysfunction, DNA fragmentation, and activation of intrinsic apoptotic pathways involving Bax and caspase-3 [7]. Our data confirmed this, as HDFs cultured in 0.1% FBS displayed increased APOPercentage™ staining and pro-apoptotic protein expression. In contrast, co-culture with ADSCs significantly reduced apoptosis, indicating a protective role likely mediated through soluble paracrine factors, in agreement with prior findings in cardiac, renal, and dermal fibroblast models [8, 24, 25].
Among the tested candidates, HGF was the most abundantly secreted factor in ADSC-conditioned media, with levels rising over time. This aligns with previous reports showing that HGF is a principal effector molecule released by ADSCs during tissue repair [26, 27]. HGF is known to bind to its receptor c-Met, a receptor tyrosine kinase expressed in multiple mesenchymal cell types including dermal fibroblasts, where it activates survival and migration pathways [28, 29]. Despite the central role of HGF in mediating fibroblast protection, our data indicate that neutralization of HGF did not completely reverse the anti-apoptotic effect of ADSCs. This observation suggests the presence of additional bioactive components in the ADSC secretome that may work synergistically with HGF. Among them, VEGF and IGF-1, which were also detected in our conditioned media, may play auxiliary roles in promoting cell survival under stress conditions. Although these factors were not the primary focus of our study, acknowledging their potential contribution adds important nuance to the interpretation of ADSC-mediated cytoprotection. Future studies dissecting the interplay among multiple secreted cytokines will provide a more comprehensive understanding of the ADSC secretome’s therapeutic efficacy. Functional blockade of HGF using a neutralizing antibody reversed the anti-apoptotic effect of ADSCs, confirming its necessity in mediating fibroblast protection.
The expression of c-Met in HDFs was confirmed at both the surface and molecular levels in our study, supporting prior transcriptomic and proteomic analyses of human skin fibroblasts [30, 31]. Furthermore, to ensure that the protective signaling via HGF was not confounded by receptor downregulation under serum stress, we examined the expression levels of c-Met under both normal (10% FBS) and reduced-serum (0.1% FBS) conditions. We found that c-Met expression was stably maintained across conditions, indicating that receptor availability was not significantly diminished during apoptotic stress. This finding strengthens the interpretation that the HGF–c-Met axis remained functionally intact and responsive, thus serving as a reliable pathway through which ADSCs confer protection. Such validation is essential, as alterations in receptor expression under stress could otherwise obscure the effects of ligand-based therapies. Inhibition of c-Met signaling led to a comparable increase in apoptosis, reinforcing the functional dependence on HGF–c-Met interactions. Notably, HGF–c-Met engagement activates a variety of downstream signaling cascades, but PI3K/AKT has been identified as the most consistent pathway promoting cell survival across multiple systems [32–34].
Our study showed that co-culture with ADSCs selectively increased phosphorylation of AKT in HDFs, without affecting the ERK1/2 or p38 MAPK pathways. This pattern of selective PI3K/AKT activation by HGF is consistent with prior work in airway epithelial cells, renal tubular cells, and fibroblasts, where it prevents apoptosis through the inhibition of pro-apoptotic Bcl-2 family members and activation of mTOR and GSK-3β [35–37]. The pharmacological blockade of PI3K/AKT by LY294002 abrogated the protective effect of ADSCs, confirming the requirement of this pathway for ADSC-mediated cytoprotection. Conversely, ERK and p38 inhibitors had no effect, suggesting that mitogen-activated protein kinases may not be involved in this specific HGF-driven context, despite their broader role in stress signaling and cell cycle regulation [38].
Collectively, our findings support a model in which ADSC-derived HGF binds to c-Met on HDFs, triggering the PI3K/AKT pathway and suppressing intrinsic apoptosis. These results provide mechanistic insights into how ADSCs contribute to dermal homeostasis and wound healing via paracrine signaling. Given the increasing interest in using cell-free stem cell therapies in regenerative medicine, our findings suggest that HGF-enriched ADSC secretome may represent a valuable therapeutic strategy for enhancing skin cell survival during tissue injury, aging, or surgical reconstruction [39].
However, our study is limited by its in vitro design. While indirect co-culture systems simulate paracrine interaction, they do not fully replicate the ECM remodeling or immune cross-talk present in vivo. Furthermore, although HGF was functionally dominant, combinatorial effects of other ADSC-derived factors such as IGF-1, VEGF, and TGF-β were not evaluated in depth. Future studies should employ in vivo wound models or 3D organotypic skin cultures, and evaluate temporal dynamics of the secretome under oxidative or inflammatory stimuli [40].
These findings provide not only mechanistic insights into the anti-apoptotic effects of ADSC-derived HGF but also highlight the clinical implications of modulating paracrine signaling in regenerative medicine. In particular, enhancing HGF–c-Met–PI3K/AKT signaling in dermal fibroblasts could be harnessed to support tissue integrity and improve outcomes in wound healing and skin rejuvenation therapies. Such strategies may be especially beneficial for patients with impaired healing capacity, including the elderly or individuals with chronic wounds. Further preclinical studies and clinical translation efforts will be necessary to evaluate the safety, efficacy, and delivery modalities of ADSC-derived factors in real-world therapeutic contexts.







