INTRODUCTION
Bone undergoes continuous resorption and regeneration processes, collectively known as bone remodeling, which have a crucial role in maintaining the balance of bone structure in the body [1]. These processes are regulated by osteoclasts and osteoblasts, and any disruption in the balance between these two cell types can lead to skeletal disorders. Osteoporosis, in particular, is a common age-related disease that primarily affects postmenopausal women [2, 3]. Osteoporosis occurs when the resorptive activity of osteoclasts surpasses the regenerative activity of osteoblasts, disrupting the balance of bone remodeling [4, 5]. Excessive osteoclast differentiation contributes to osteoporosis development, which implies that inhibition of osteoclast differentiation is a potential therapeutic approach [6]. Bisphosphonates, a common treatment for osteoporosis, are associated with side effects, such as jawbone degradation [7]. Consequently, there has been growing interest in researching natural substances that may inhibit osteoclast differentiation to improve treatment outcomes [6, 8–12].
Osteoclasts are large multinucleated cells formed by fusing monocyte precursors from the hematopoietic stem-cell lineage [13]. Differentiation of osteoclasts requires key factors, including macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-Β ligand (RANKL) [14]. Binding of RANKL to its receptor RANK activates various signaling pathways, leading to activation of nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), the final transcription factor for osteoclast differentiation, through c-Fos and nuclear factor kappa-light-chain-enhancer of activated B. This activation promotes the expression of genes associated with osteoclasts [15–17].
Recent studies in our laboratory have shown that extracts from the fruiting body of Lentinula edodes, commonly known as shiitake mushrooms, can inhibit osteoclast differentiation by suppressing NFATc1 expression [18]. However, the medicinal properties of mushrooms can vary depending on both the part of the mushroom used and drying method used [19–21]. The different parts of the mushroom, such as the cap, stipe, and mycelium, contain varying levels of bioactive compounds that contribute to their medicinal properties [22–24]. Moreover, the drying method used can significantly influence the chemical composition and medicinal potential of shiitake mushrooms. Techniques, such as air drying, freeze-drying (FD), and oven drying, affect the stability and concentration of bioactive compounds, including polysaccharides, terpenoids, and phenolics [20]. Understanding how mushroom parts and drying methods influence osteoclast differentiation is crucial for enhancing their therapeutic efficacy.
In this study, we investigated the effects of different drying methods on the bioactive properties of shiitake mushroom caps and stipes, focusing on their ability to inhibit osteoclast differentiation. Using cell-based assays and osteoporosis-induced zebrafish models, we aimed to elucidate how the drying process influences the medicinal properties of these mushroom parts and evaluate their potential as therapeutic agents for osteoporosis.
MATERIALS AND METHODS
The fruiting bodies of L. edodes (strain Chamaram) were separated into caps and stipes, sliced thinly, and dried using three methods: FD, natural drying (ND), and hot air drying (HAD) for 72 hours. The dried samples were dissolved in water and subjected to ultrasonic treatment for 1 hour using an ultrasonic bath (DAIHAN-Sci, Seoul, Korea). After filtration, the extracts were concentrated using a rotary vacuum evaporator RV 8 (IKA®-Werke GmbH & Co. KG, Staufen im Breisgau, Germany).
Osteoclast precursor (OCP) cells were prepared as described previously [25]. Briefly, bone marrow cells were harvested from the tibiae and femora of 6-week-old ICR male mice. The cells were cultured in α-minimum essential medium containing 10% FBS and 5 ng/mL of M-CSF (IKIM Bio, Cheongju, Korea) for 16 hours. Non-adherent cells were collected and further cultured in α-MEM with 10% FBS and M-CSF (30 ng/mL) for 3 days. After removing floating cells, adherent OCP cells were used for differentiation assays. OCPs were treated with 30 ng/mL of M-CSF (IKIM Bio) and 100 ng/mL of RANKL (IKIM Bio) in the presence of shiitake mushroom extracts derived from caps or stipes, prepared using three drying methods (FD, air-drying, and HAD), at two concentrations (5 µg/mL and 10 µg/mL). The cells were cultured in 48-well plates for 3 days. On the third day, the cells were fixed with 3.7% formaldehyde and stained for tartrate-resistant acid phosphatase (TRAP) using an acid phosphatase leukocyte kit (Sigma-Aldrich, St. Louis, MO, USA). TRAP-positive multinucleated cells were counted under a light microscope.
To evaluate the effects of shiitake mushroom extracts on cell proliferation, OCP cells were treated with mushroom extracts (5 µg/mL and 10 µg/mL) derived from caps and stipes using different drying methods. Cell viability was assessed using the Cell Proliferation Kit 1 (Roche Diagnostics, Mannheim, Germany) by performing MTT assays on days 1, 2, and 3.
Whole-cell lysates were prepared using a lysis buffer (25 mM Tris, pH 7.9, 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 5% glycerol, protease inhibitor cocktails, 1 mM sodium orthovanadate, and 2.5 mM sodium pyrophosphate). Protein concentrations were measured using a Protein Assay Kit (iNtRON Biotechnology, Seoul, Korea). Lysates were separated by SDS-PAGE and transferred to PVDF membranes (GE Healthcare, Freiburg, Germany). Western blotting was performed using the following antibodies: NFATc1 and p65 (Santa Cruz, CA, USA), β-actin (Sino Biological, Beijing, China), and c-Fos (Millipore, Burlington, MA, USA).
The samples were filtered using a 0.45 µm pore size membrane filter and then analyzed via Accelar UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic separation was performed using a ACQUITY UPLC column (BEH C18: 2.1 × 50 mm, 1.7 μm) with 0.4 mL/min flow rate. A gradient elution was achieved using two mobile phases consisting of 0.1% (v/v) formic acid (mobile phase A) and acetonitrile with 0.1% (v/v) formic acid (mobile phase B). Quantification was based on a standard curve prepared with denatonium (Sigma-Aldrich).
Zebrafish were maintained in a recirculating system at 28°C under a 14-hour light/10-hour dark cycle and fed brine shrimp three times daily. Fertilized eggs were obtained from mating adult zebrafish and incubated at 28°C under the same light/dark conditions. Larvae at 10–13 days post-fertilization (dpf) were treated with shiitake mushroom extracts (5 µg/mL) or prednisolone (PS; 25 µM). Whole-mount skeletal staining was performed at 13 dpf [26].
Alizarin red staining of zebrafish larvae was conducted following established protocols. Larvae at 13 dpf were fixed in 10% neutral buffered formalin and depigmented using 3% H2O2. Samples were stained with 1 mg/mL alizarin red in 1% KOH (pH 4.28) and cleared in increasing concentrations of glycerol (20%, 40%, and 60% in 1% KOH). Images of stained vertebrae were captured using a stereo microscope (SMZ18, Nikon, Tokyo, Japan). Bone mineral density was quantified using ImageJ software.
RESULTS
Our recent study found that a FD extract of the fruiting bodies of L. edodes inhibited osteoclast differentiation [18]. To further identify which part is responsible for this effect, the fruiting bodies were separated into caps and stipes, FD, and extracted (Fig. 1A). To evaluate the inhibitory effects of these extracts on osteoclast differentiation, OCP cells were treated with M-CSF and RANKL, along with the mushroom extracts at two concentrations (5 µg/mL and 10 µg/mL) for 3 days. Subsequent formation of TRAP-positive osteoclasts indicated that the stipe extracts significantly inhibited osteoclast differentiation, whereas the cap extracts had minimal effects (Fig. 1B and C). Given that the bioactivity of the extracts could vary depending on the drying method, ND and HAD methods were used to further process the cap and stipe. The resulting extracts were tested for their effects on osteoclast differentiation. The HAD cap extracts exhibited slight inhibitory activity, whereas the ND cap extracts had almost no effect on osteoclast differentiation. In contrast, the stipe extracts consistently inhibited osteoclast differentiation regardless of the drying method (Fig. 1B and C).
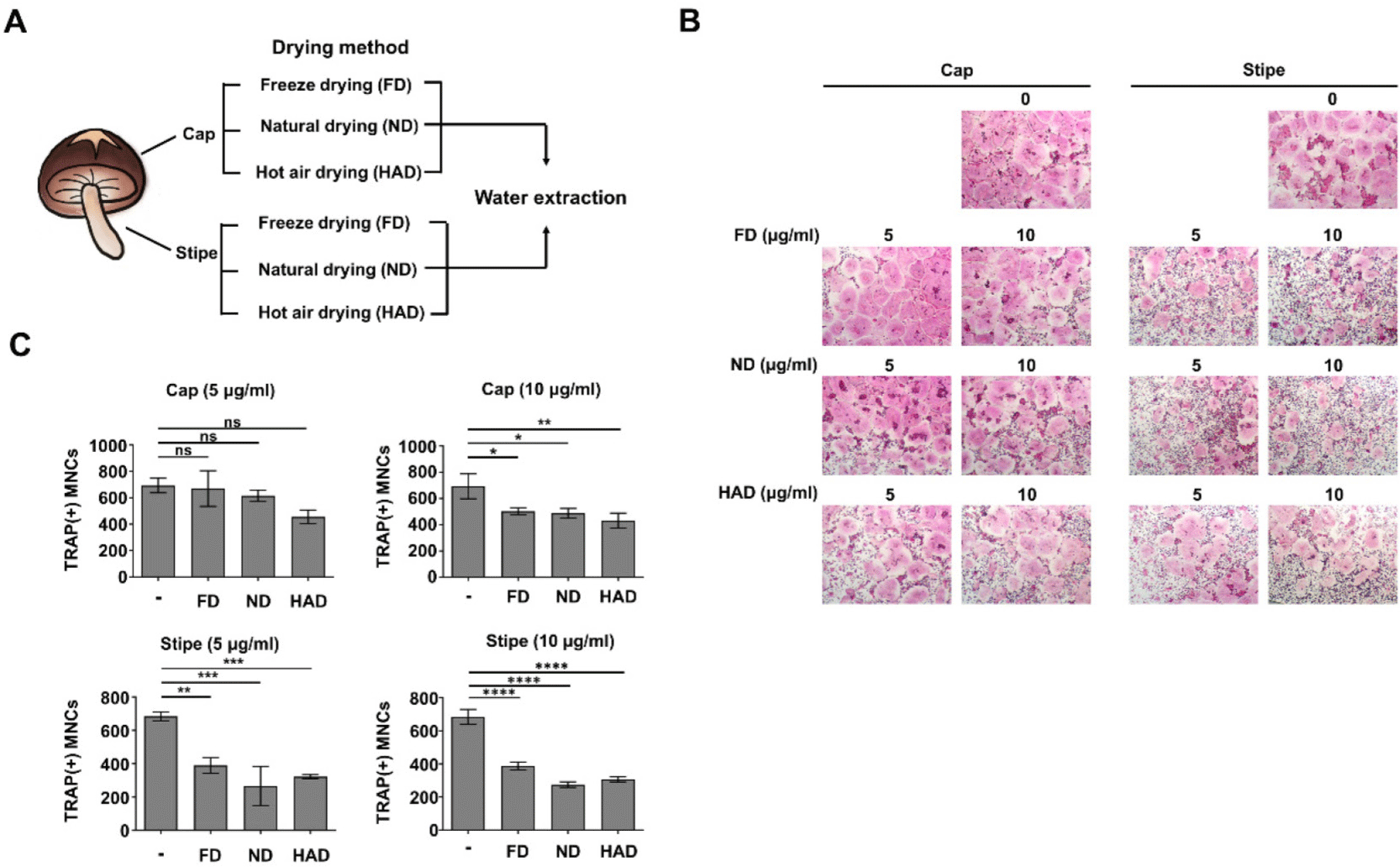
We evaluated OCP cell proliferation to determine if the bioactive compounds in the shiitake mushroom extracts inhibited OCP cell differentiation directly through antiosteoclastogenic activity or indirectly by inhibiting suppression. Shiitake mushroom cap and stipe extracts prepared by different drying methods were treated with M-CSF at two concentrations (5 µg/mL and 10 µg/mL) for 3 days. There were no significant differences in OCP cell proliferation between the treated groups and untreated control (Fig. 2). These findings suggested that the shiitake mushroom stipe extracts inhibit OCP cell differentiation through bioactive compounds with direct antiosteoclastogenic activity rather than by affecting cell proliferation.
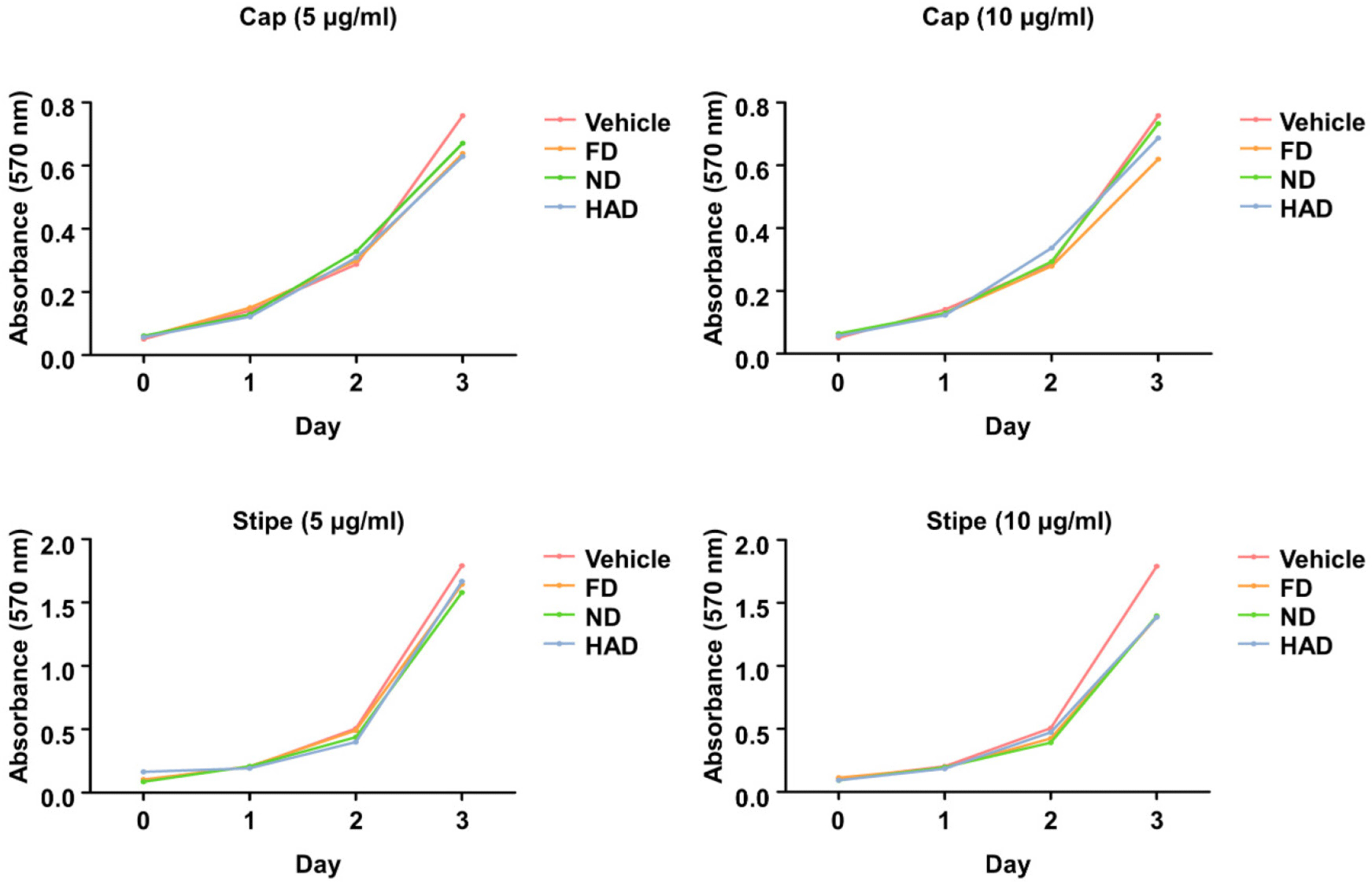
We next investigated the effects of shiitake mushroom cap and stipe extracts prepared using different drying methods on the protein expression of NFATc1, a master transcription factor involved in osteoclast differentiation (Fig. 3A). Stipe extracts dried by all three drying methods significantly reduced NFATc1 protein expression, whereas the cap extracts, regardless of the drying method, had no effect. Given that our recent study [27] identified denatonium as a key molecule in L. edodes responsible for suppressing osteoclast differentiation and inhibiting NFATc1 expression, we quantified the presence of denatonium in the extracts by performing high-performance liquid chromatography. Denatonium was detected exclusively in the stipe extracts, but it was absent in the cap extracts (Fig. 3B). These findings suggest that bioactive compounds or one compound, such as denatonium, present in the shiitake mushroom stipe extracts interfere with the transcriptional regulation of NFATc1. This interference leads to reduced NFATc1 protein production, thereby impairing RANKL signaling and suppressing osteoclast differentiation.
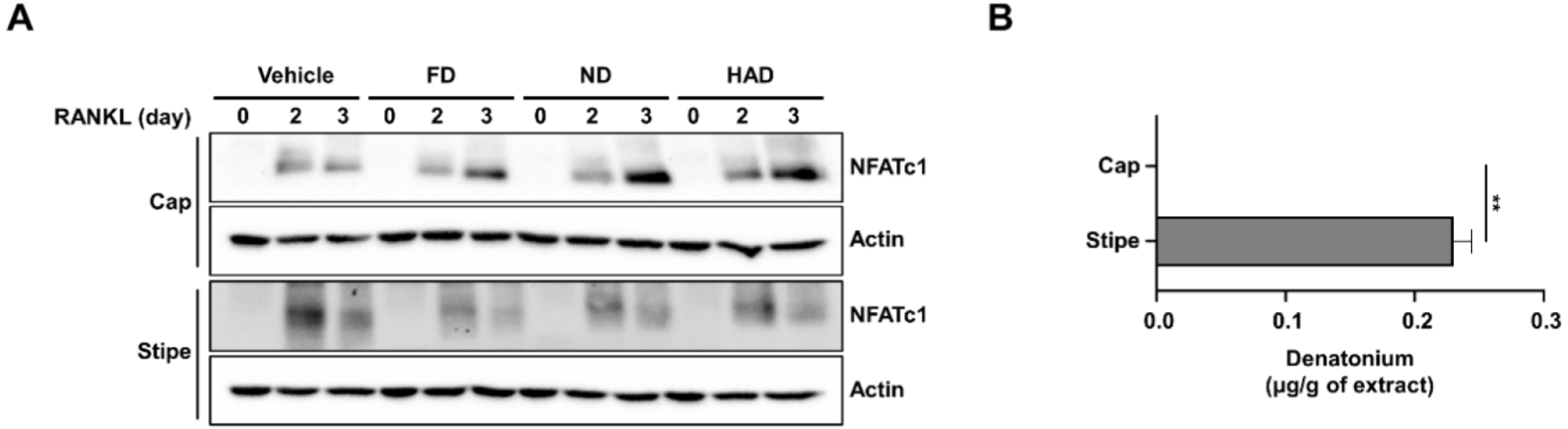
To investigate the in vivo efficacy of shiitake mushroom stipe extracts prepared by the different drying methods in osteoporosis, a transparent zebrafish larva model was used. Osteoporosis was induced in 10-day-post-fertilization (10 dpf) zebrafish larvae by treating them with PS, establishing an osteoporosis model system. Shiitake mushroom stipe extracts (5 µg/mL) from different drying methods were cotreated with PS for 3 days, followed by bone staining of the zebrafish larvae (Fig. 4). The results showed that PS treatment reduced bone mineralization, confirming osteoporosis induction. However, treatment with the shiitake mushroom HAD extracts restored bone mineralization to levels comparable to those observed in the control group whereas FD and ND extracts showed only modest improvement. These findings indicate that shiitake mushroom HAD stipe extracts can mitigate PS-induced bone mineralization loss, supporting the potential for therapeutic effects on bone formation in osteoporosis.
DISCUSSION
This study investigated the effects of shiitake mushroom cap and stipe extracts, processed by three drying methods, on osteoclast differentiation and bone formation. The findings indicated that shiitake mushrooms can be used to produce a natural potentially therapeutic agent for osteoporosis in humans. Our results demonstrated that stipe extracts, regardless of the drying method used, effectively inhibited osteoclast differentiation by suppressing NFATc1 expression. Notably, the cap extracts showed minimal inhibitory effects on osteoclast differentiation, highlighting the stipe as a more potent source of bioactive compounds. This difference underscores the importance of investigating specific mushroom parts for their unique bioactive properties.
Our study further investigated the mechanism underlying the antiosteoclastogenic effects of shiitake mushroom stipe extracts. By assessing the proliferation of OCP cells, we confirmed that the inhibition of osteoclast differentiation was not attributable to reduced cell proliferation. Instead, the stipe extracts directly suppressed the expression of transcription factors, such as NFATc1. These results indicate that denatonium and potentially other bioactive compounds directly disrupt RANKL signaling. This targeted interference highlights the potential of stipe extracts as a therapeutic approach for mitigating osteoclast activity in diseases, such as osteoporosis.
Interestingly, the drying method did not significantly alter the ability of the stipe extracts to inhibit osteoclast differentiation, as indicated by the consistent suppression of NFATc1 protein expression across all three methods. However, in vivo studies using zebrafish larvae revealed varying degrees of bone restoration. Specifically, both the FD and ND stipe extracts resulted in modest bone restoration, whereas the HAD stipe extracts led to statistically significant recovery. This discrepancy may be attributed to several factors. The HAD extracts might contain bioactive compounds that more effectively promote osteoblast differentiation and bone mineralization in vivo. Additionally, the drying process used for HAD extracts could preserve or enhance specific molecular components that interact more favorably with the zebrafish larvae model. Further studies are needed to better understand the underlying mechanisms and the specific contributions of the HAD extracts in promoting bone restoration. Nevertheless, the fact that HAD stipe-extract treatment restored bone mineralization in PS-treated zebrafish larvae demonstrates its efficacy in mitigating osteoporosis-induced bone loss. These findings underscore the potential of HAD extracts to promote bone formation in a physiologically relevant model, highlighting their promise as a natural alternative to current osteoporosis treatments, such as bisphosphonates, which are associated with adverse side effects [7].
The differential bioactivity observed between the cap and stipe of shiitake mushrooms underscores the importance of part-specific analyses in future research. The stipe appears to contain unique compounds, such as denatonium, which are critical for its antiosteoclastogenic effects. Additionally, the stability of these bioactive compounds across various drying methods warrants further chemical analyses to identify the specific molecules responsible for the observed effects. Such research could enable the development of standardized extraction and drying methods to maximize therapeutic efficacy.
Despite these promising results, several study limitations should be considered. Although the zebrafish model provides a robust platform for in vivo analysis, validation in mammalian models is essential to establish the translational potential of these findings. Detailed molecular studies also are needed to fully elucidate the signaling pathways modulated by the stipe extracts.
In conclusion, the study results highlight the potential of shiitake mushroom stipe extracts, processed by various drying methods, as a natural therapeutic option for osteoporosis. By inhibiting osteoclast differentiation and promoting bone formation, these extracts offer a promising alternative to current pharmacological treatments.







