INTRODUCTION
Antimicrobial resistance (AMR) is the process through which bacteria, fungi, viruses, parasites, and other microorganisms develop resistance to antimicrobials agents. Bacteria in humans or animals which develop AMR can no longer be treated with antibiotics or other antimicrobial drugs [1]. Currently, the rapid increase in AMR represents a global issue affecting both human and animal health [2]. For example, the incidence of AMR in humans leads to approximately 25,000 fatalities annually in Europe, along with nearly 2.5 million excess hospitalization person-days [3]. Similarly, the 2019 Antibiotic Resistance Threats Report by the Centers for Disease Control and Prevention highlighted the severity of antibiotic resistance in the US, estimating over 2.8 million cases per year, leading to more than 35,000 deaths. China also faces approximately 60,000 deaths annually due to AMR [4]. The World Health Organization (WHO) estimates that AMR causes 700,000 deaths annually. Projections have further indicated that this number could increase to 10 million by 2050, if appropriate measures are not taken to address this issue [5]. In the case of animals, the rapid rise in AMR not only causes an increased mortality rate, but also contributes to an annual decline in global livestock production ranging from 2.6% to 7.5% by 2050. [6].
The rapid increase in AMR has resulted from the overuse of antimicrobials in human and animal populations [7, 8]. For example, the frequent and unnecessary use of antibiotics to treat mild diseases such as skin injuries, respiratory illnesses, and urinary tract infections has accelerated the emergence of AMR in humans [9, 10]. In domestic animals, the overuse and misuse of antimicrobials have been common practices not only for treating infectious diseases, but also for increasing productivity, subsequently raising AMR risks [11].
Recently, the overuse of antimicrobials has become a concern in companion animals [12]. Overuse, particularly in treating common infections, such as urinary tract infections, can lead to the emergence of resistant bacterial strains in companion animal populations [13]. Similarly, prolonged antibiotic use for dermatological conditions, such as pyoderma, may contribute to the development of AMR, potentially reducing the effectiveness of subsequent treatments [14]. Additionally, the inappropriate use of last-resort antibiotics in companion animals, such as vancomycin and polymyxin, which are critical for treating severe infections, not only complicates clinical management, but also plays a significant role in the emergence of AMR [15]. For example, the misuse of vancomycin, which is specifically reserved for severe gram-positive infections, can lead to its ineffectiveness in cases with proper diagnostic justification. This misuse may lead to the development of vancomycin-resistant Staphylococcus aureus and enterococci strains [16].
Regular surveillance of antimicrobial use in companion animals could be beneficial in reducing overuse and the associated AMR risks, as demonstrated by the surveillance programs for industrial animals. For example, since 2010, sales of veterinary antimicrobial agents in Europe have been tracked by the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). A 15% reduction in the overall use of antibiotics in animals was first reported in ESVAC reports covering 26 EU countries between 2010 and 2012; these reports account for approximately 95% of the animal population in the EEA/EU region [17]. Similarly, Germany instituted a surveillance system in 2011 to track the sales of antimicrobials for veterinary use, which led to a notable decline in sales volume over eight years, decreasing by 58%, from 1,706 tons to 722 tons [18]. However, there is currently no regular surveillance system for antimicrobial use in companion animals in South Korea. Considering the practical difficulties of implementing a universal surveillance system that includes all veterinary clinics, a sentinel surveillance system should be developed in the future. Pilot investigations are important for providing the baseline information necessary to design a representative monitoring system. In this regard, we performed preliminary investigations of antimicrobial use in small animal clinics, especially focusing on companion dogs and cats due to their dominance in companion animal population.
MATERIALS AND METHODS
This study collected and analyzed recorded information on antibiotic use from multiple small animal veterinary clinics in South Korea. Initially, candidates were recruited through the Korean Animal Hospital Association (KAHA), a professional association of veterinary clinics in South Korea. A total of 17 veterinary clinics expressed interest in the study (total number of veterinary clinics for companion animals in South Korea is about 3,000). Following an assessment of their characteristics (size and location) and the integrity of their data storage and records, 12 hospitals were finally included in this study. The excluded 5 hospitals showed low data quality and completeness. Among the 12 selected hospitals, six hospitals are located in metropolitan area (the numbers of primary and secondary hospitals were four and two, respectively) and the other six are located in non-metropolitan area (the numbers of primary and secondary hospitals were four and two, respectively). Considering that only preexisted medical records were used in this study, review by the Institutional Animal Care and Use Committee was not required.
All data were collected from electronically stored records at participating veterinary clinics. Prior to data collection, the director of each participating clinic received in-person 10-min long pre-education, completed a consent form for data collection and utilization, and completed a survey regarding antibiotic stewardship. The researchers then conducted individual visits to the participating clinics to assess the adequacy of recording and storing antibiotic use data in the electronic medical record system before proceeding with data collection.
The data collected included patient signalment, clinical notes, relevant diagnostic tests, and antibiotic prescriptions (generic name, brand name, dosage, and route of administration) over a three-year period from January 2018 to December 2020. Based on the medical records, the researchers categorized the purpose of antibiotic use (treatment of infection, prophylaxis [preventive purposes prior to surgeries], or use for reasons unrelated to antimicrobial activity) using the following key associated words (Table 1). If the intended reason was unspecified or ambiguous, it was excluded from the data. Cases with multiple purposes (e.g., skin infection with gastrointestinal infection) were categorized by checking multiple boxes, and cases where it was unclear which antibiotic was prescribed for which purpose were not included in the database.
Antibiotics included systemic antibiotics (oral and injectable) and topical antibiotics (ophthalmic and dermatologic). The active ingredient content of the antibiotic prescribed or administered to the patient was calculated based on the active ingredient content of the product, with both the ingredient name and product name identified. If a patient was prescribed multiple antibiotics, all relevant details were recorded.
The identification and categorization of the medical records were based solely on the information obtained, without consideration of the level of evidence for bacterial infection or judgments about the appropriateness of the antibiotics prescribed or administered.
The data were accessible only to the submitting veterinary clinics and the researchers, and no data were collected on identifiable clients, animals, or prescribers. Analyses and calculations were performed using SPSS version 24 (IBM, Armonk, NY, USA) and summarized as frequencies (n) and percentages (%), with missing data excluded.
RESULT
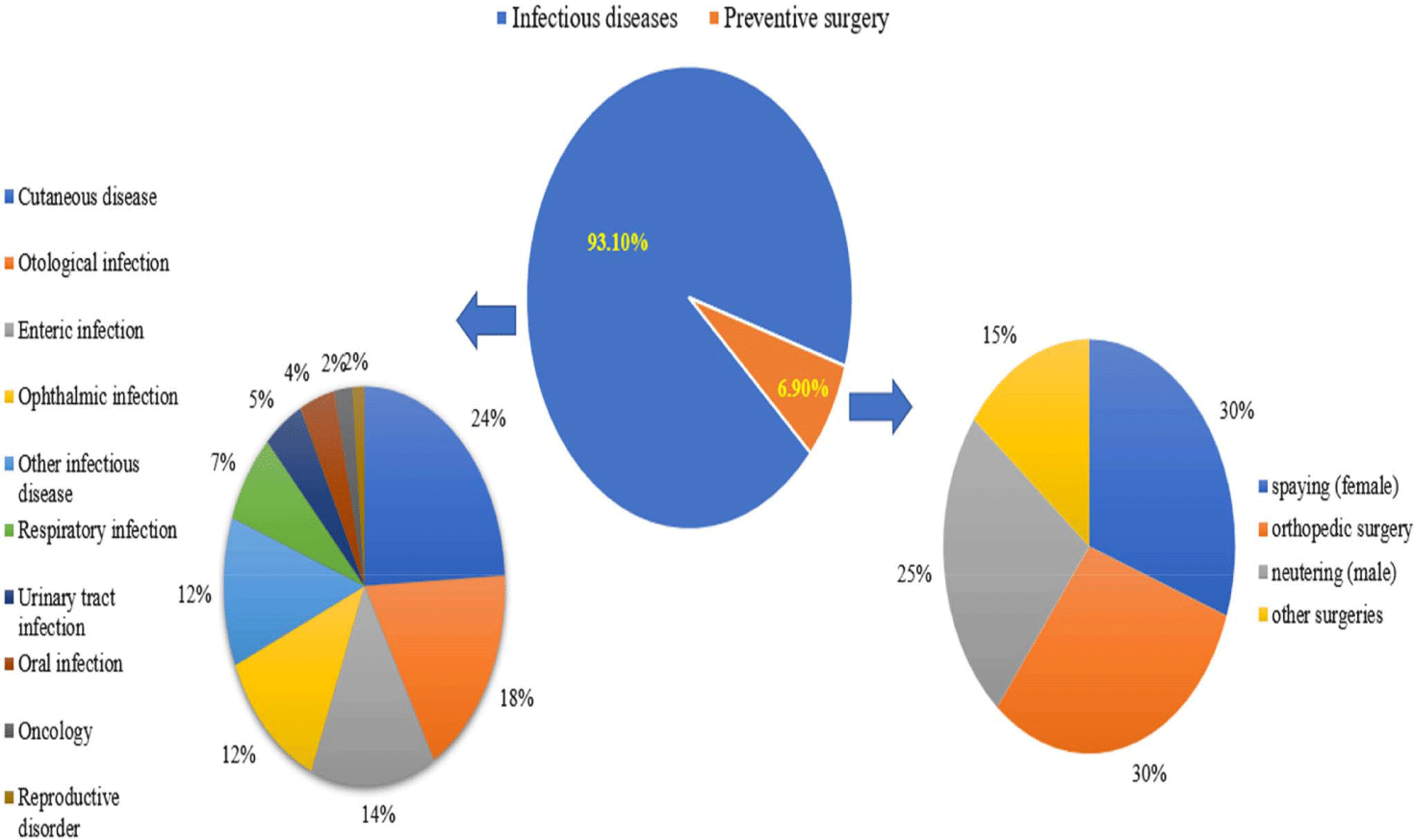
According to a three-year survey of antibiotic prescription records at 12 small animal hospitals, 93.1% of all antibiotic prescriptions were provided for the treatment of infectious diseases, while 6.9% were for prophylaxis (Fig. 1). Regarding disease treatment, the highest proportion of prescriptions was for cutaneous diseases (Supplementary Table S1, Fig. 2), whereas for prophylaxis, the highest proportion was for neutering procedures (Supplementary Table S2, Fig. 2).
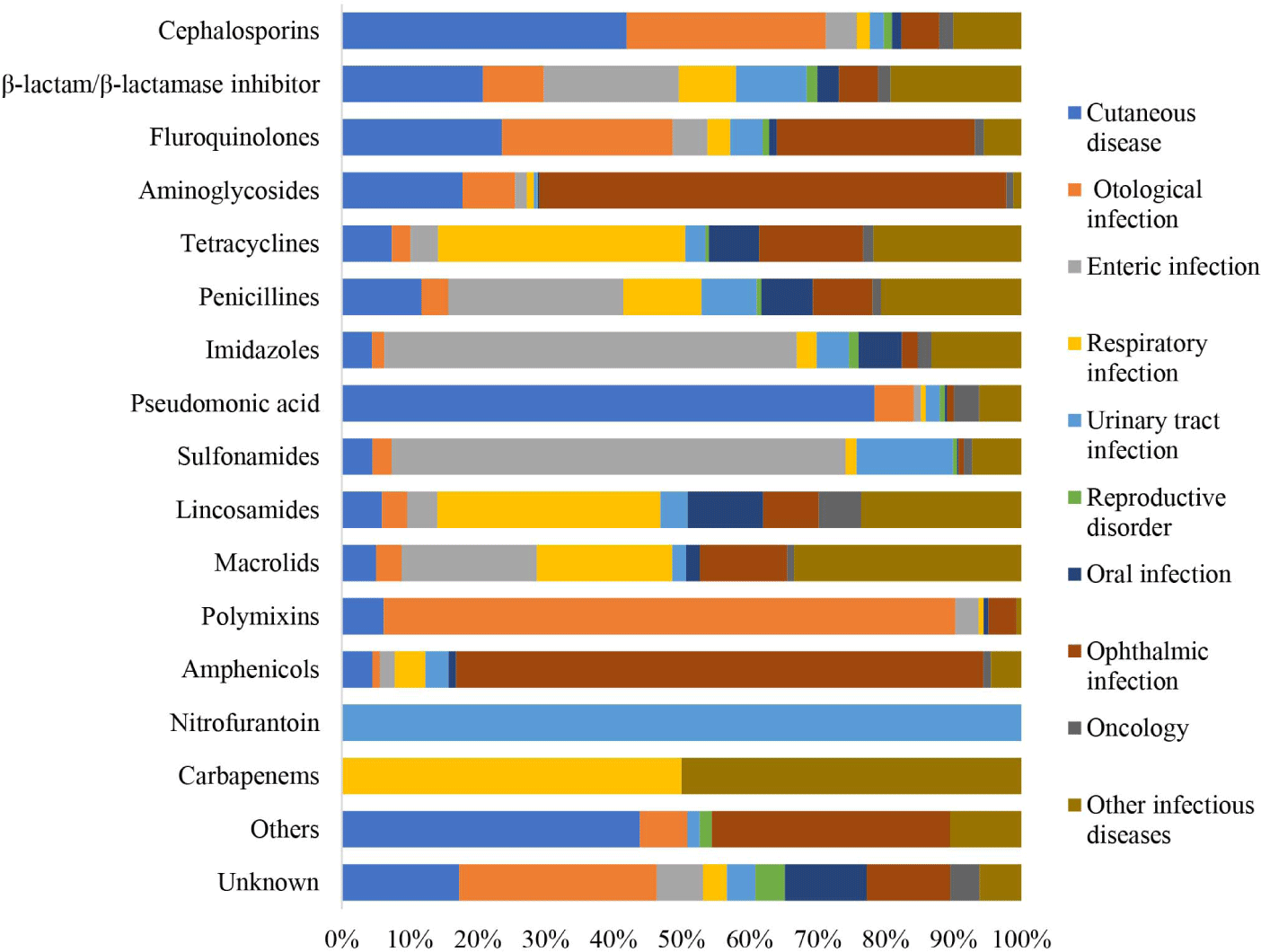
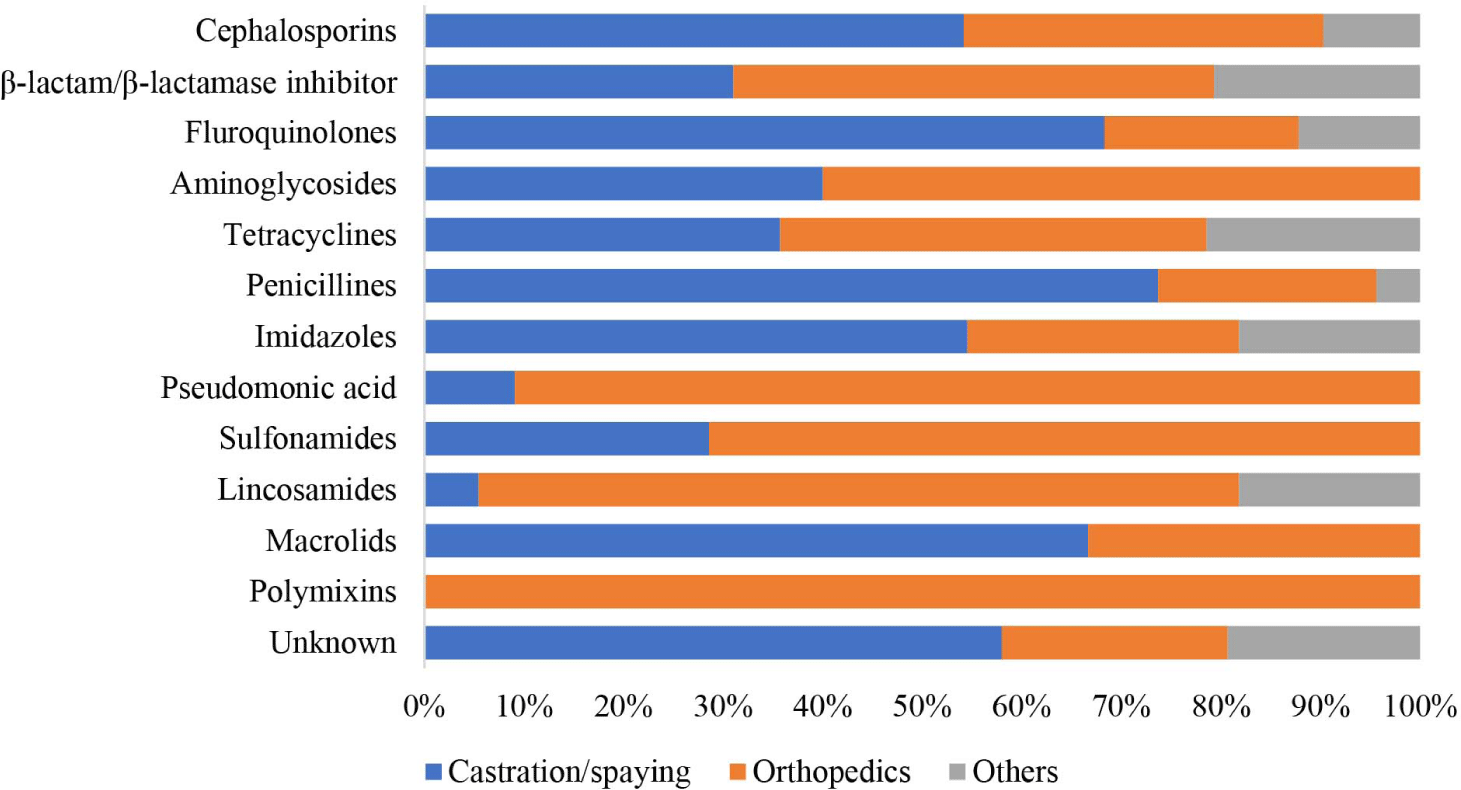
Overall, the findings of this study indicated that nitrofurantoin was mainly prescribed for urinary tract infections (100%), polymyxins for otological infections (83.0%), amphenicols for ophthalmic infections (77.5%), pseudomonic acid for cutaneous diseases (75.8%), sulfonamides for enteric infections (66.3%), and carbapenems for respiratory infections (50%). Lincosamides were the preferred type of antibiotics used in cases of oral infections, accounting for 10.5% of cases, and they are also utilized in oncology at a rate of 5.9%. Macrolides were predominantly used for the treatment of other infectious diseases (33.1%; Supplementary Table S1, Fig. 2). Finally, for prophylaxis, such as castration/spaying, orthopedics, and other surgeries, penicillin was used in 73.7%, 22%, and 4.3%, of cases respectively (Supplementary Table S2, Fig. 3).
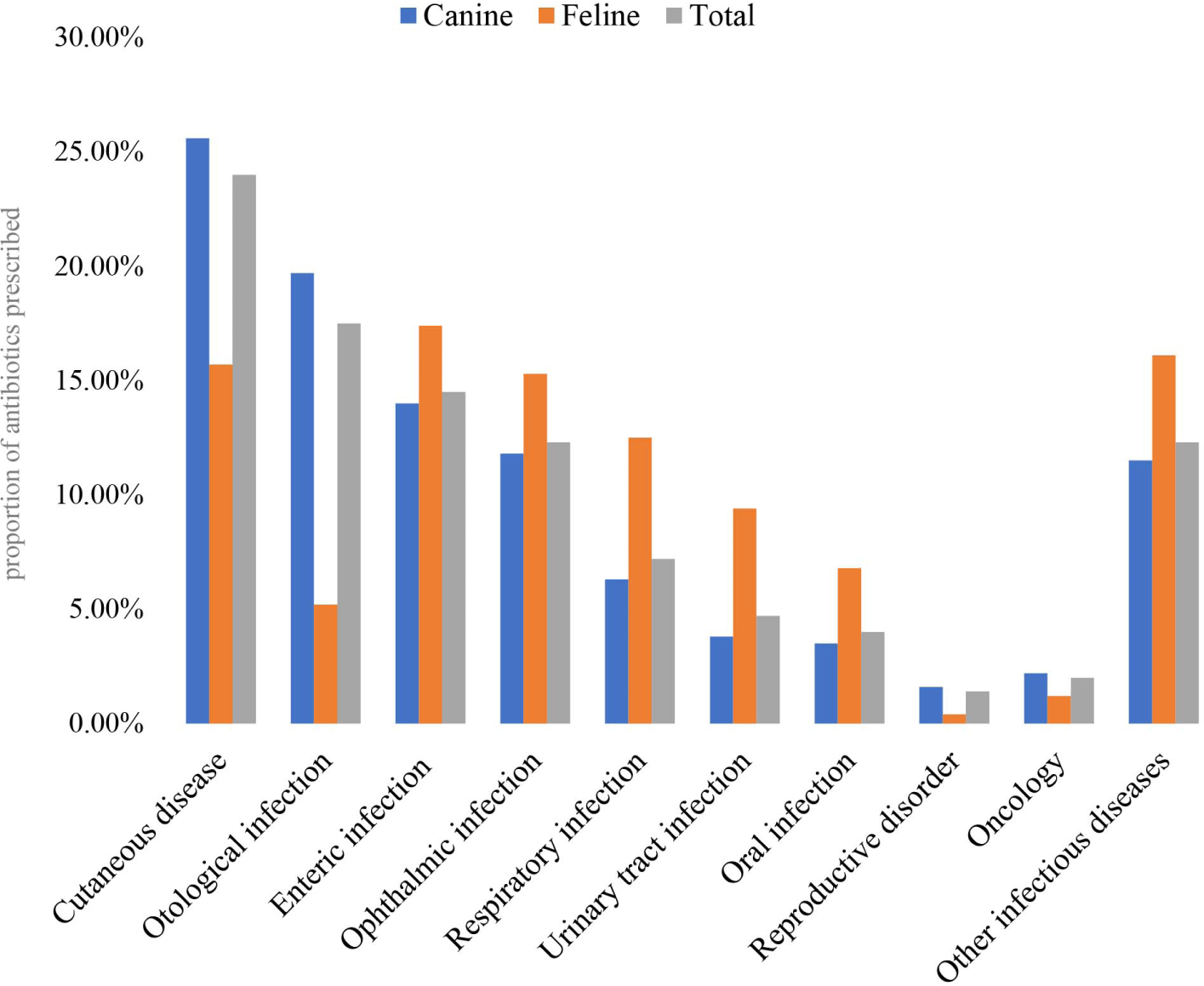
An illustrative comparison of the proportions of antibiotic prescriptions between dog and cat types/diseases is provided in Fig. 4. The results show that Antibiotics were predominantly used in felines rather to canines for enteric infections (17.4%), respiratory diseases (15.3%), urinary tract infections (12.5%), reproductive disorders (9.4%), oral infections (6.8%), and other infectious diseases (16.1%). In contrast, for cutaneous diseases (25.6%), otological infections (19.7%), oncology (2.2%), and ophthalmic infections (1.6%), veterinarians prescribed a higher proportion of antibiotics to canine than feline patients (Supplementary Table S3).
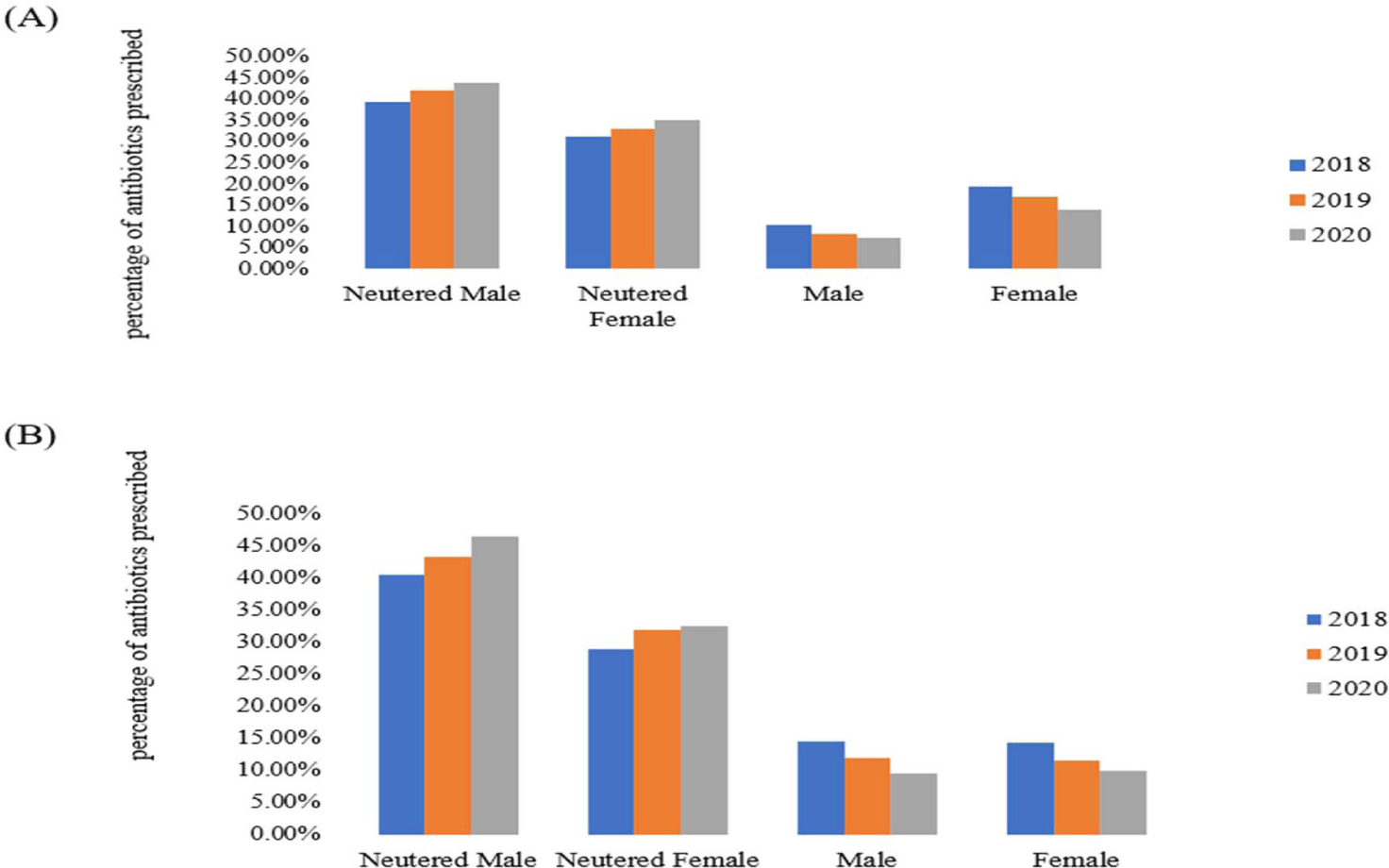
From 2018 to 2020, a higher proportion of antibiotics was prescribed to neutered males than to other dogs and cats, including neutered females, intact males, and intact females (Fig. 5). Over time, the proportion of antibiotic use gradually increased in both neutered males and females. In contrast, the proportion of antibiotic use decreased in intact males and intact females across the years. In 2018, 10.3% of antibiotics were used in male dogs and 19.2% in female dogs, but these proportions decreased to 7.4% and 13.9%, respectively, by 2020 (Supplementary Table S4). A similar pattern was observed in cats.
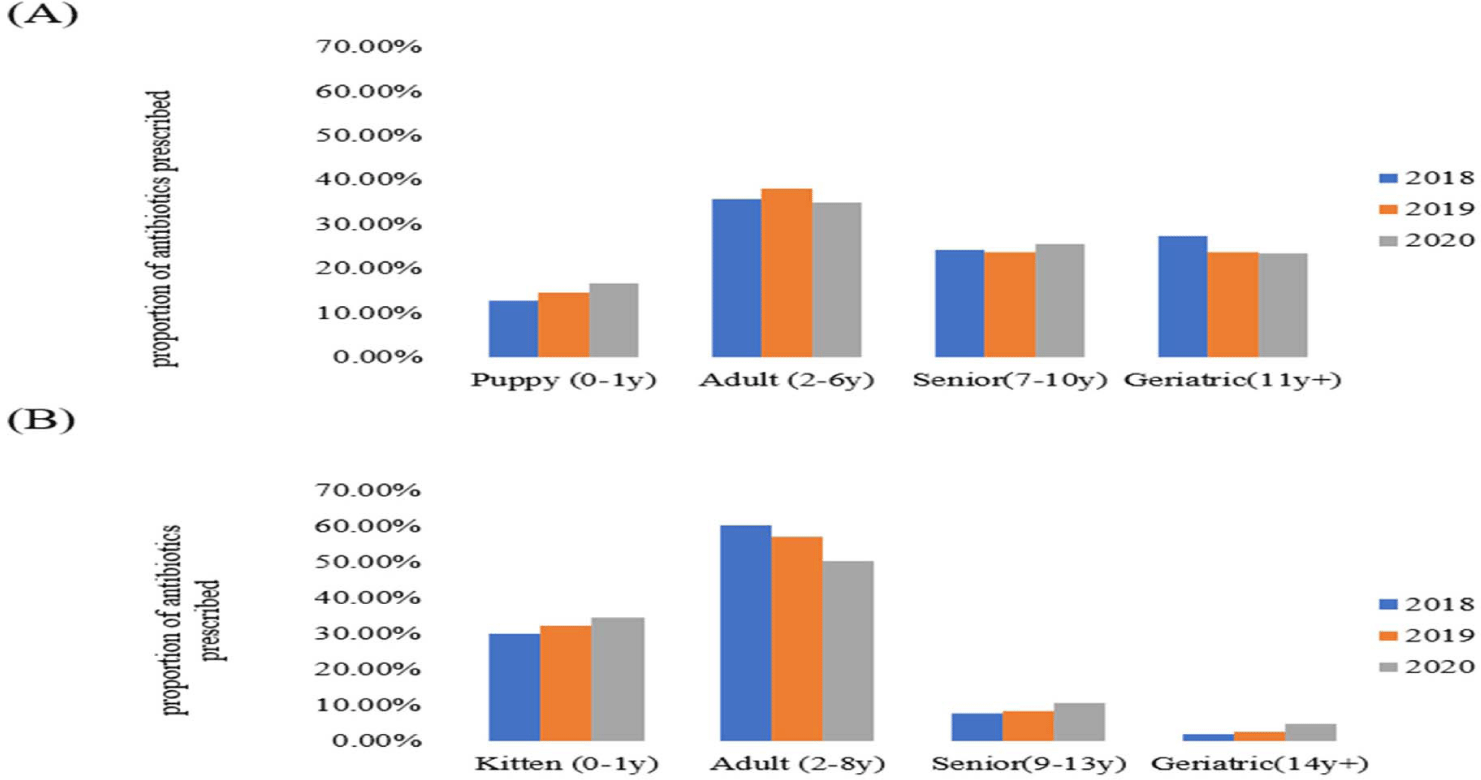
The proportion of antibiotic prescriptions in companion animals also differed among age groups between 2018 and 2020. The highest proportion of antibiotic prescriptions was observed in the adult population of dogs and cats (Fig. 6), reaching 38% in 2019 for dogs, and 60.2% in 2018 for cats. Conversely, the lowest antibiotic prescription proportions were 12.7% and 1.9% in puppies and geriatric pets, respectively, both in 2018 (Supplementary Table S5).
DISCUSSION
This study explored the use of antibiotics in companion animals across South Korean small animal clinics from 2018 to 2020, focusing on prescription trends, purposes, and variations based on species, sex, and age groups. Veterinarians commonly prescribe antibiotics both to treat infection and for prophylaxis, with a notable emphasis on cutaneous diseases for treatment and neutering procedures for prophylaxis. Specific antibiotics are recommended for the treatment of various conditions, reflecting a tailored therapeutic approach. In the present study, different proportions of antibiotic prescriptions were observed depending on the species, age group, and sex of the companion animals, indicating differences in veterinary antibiotic practices.
Our results showed that the proportion of use of antibiotic agents for the treatment of cutaneous diseases, otological infections, and enteric infections was greater than that for other infectious disorders and preventive surgeries where antibiotics are commonly used. One potential explanation for the higher proportion of antibiotic use is the higher prevalence of these specific diseases in companion animals. In a previous descriptive epidemiological study in South Korea, the most common diseases among companion dogs were cutaneous diseases (17.7%), digestive diseases (12.26%), and otological diseases (10.4%), which were higher than the others [19].
The usual prescription practices of veterinarians could also contribute to the higher proportion of antibiotic use for cutaneous, otological, and enteric infections. Antibiotics are empirically prescribed for these diseases without a confirmatory diagnosis, because they require a time delay for treatment. According to one previous study [20], 85% of veterinary practitioners engage in empirical treatment of bacterial disorders, with only 15% reporting the use of confirmatory laboratory tests. Similar trends have also been observed in New Zealand and Australia, where this practice is prevalent [21, 22]. Moreover, veterinarians sometimes face pressure from pet owners to prescribe antibiotics, even though they are not necessary for medical purposes [23, 24].
This study also revealed a higher proportion of antibiotic use in adult dogs (aged 2–6 years) and cats (aged 2–8 years). A possible reason for this is that adult dogs and cats are more likely to be in contact with infectious diseases, meaning they are more likely to require antibiotic therapy. One study reported that adult dogs (> 18 months) had a higher incidence of infectious diseases than puppies (0–7 months) and adolescents (8–18 months), although this study did not include geriatric dogs [25]. Another study found that adult cats had a higher prevalence of infectious diseases, with 60% affected by feline calicivirus compared to 7% of juvenile cats under six months old, and 84% affected by Toxoplasma gondii compared to 13% of juvenile cats [26].
The proportion of prescriptions for cutaneous diseases and otological infections was higher for dogs than for cats. From a biomedical perspective, there may be several explanations for this phenomenon. Firstly, cutaneous diseases are more prevalent in dogs than in cats, and dogs are more likely to develop conditions such as demodicosis, dermatophytosis, and food allergic dermatitis [27]. These differences can be attributed to species-specific immune responses. For example, dogs frequently experience hypersensitivity reactions to environmental allergens, which can result in allergic dermatitis, whereas cats can experience hypersensitivity reactions to ectoparasites, such as fleas, which can cause dermatological manifestations [28, 29]. Furthermore, the higher susceptibility to specific skin diseases in dogs than in cats may be due to differences in glandular composition and skin structure [30]. In addition, dogs are more susceptible than cats to auditory infections, such as otitis externa, owing to their specific anatomical and physiological characteristics. These include long ear canals, floppy ears, and a tendency to retain moisture, all of which create an environment conducive to bacterial infections [31, 32].
One primary limitation of this study is the small number of clinics, which affects its representativeness and generalizability to broader populations. By including a limited number of small animal clinics, this study may not capture diverse prescription practices and antibiotic usage patterns across different regions or healthcare settings in South Korea. Another limitation is that the study only captured the proportion of antibiotic prescriptions, without considering the actual amount of antibiotics prescribed, or utilizing standardized metrics, such as Defined Daily Doses (DDD) or Days of Therapy (DOT) indicators. These indicators provide valuable insights into the volume and duration of antibiotic use, which are crucial for assessing appropriateness and monitoring trends over time.
This study offers original insights into the factors influencing antibiotic use patterns across various settings, including those related to companion animals. We explored the correlation between increased proportion of antibiotic use in specific age groups and veterinary practices with the higher prevalence of infections such as cutaneous, otological, and enteric infections. Our analysis also delved into the distinctions between dermatological and auditory infections in dogs and cats, underscoring the importance of considering species-specific biological factors in diagnosis and treatment. Future studies should prioritize larger-scale, diverse studies, incorporate quantitative measures of antibiotic use, and account for species-specific factors in veterinary medicine in order to improve antibiotic prescribing practices in pet animals and antimicrobial stewardship. These initiatives will effectively combat overuse of antibiotics while also improving patient outcomes.