INTRODUCTION
Artificial insemination (AI) is extensively utilized to enhance reproductive performance and achieve higher fertility rates in the current swine industry [1]. Typically, diluted boar semen used for AI is stored for 1 to 5 days at 15°C–20°C, and spermatozoa may be subjected to several factors that adversely affect their quality, such as bacterial contamination and oxidative stress during storage [2, 3]. Therefore, antioxidants and antibiotics are commonly added to the diluents to maintain sperm quality during sperm storage [4]. Previous studies have found the prospective utilization of organic products such as plant extracts as alternatives to the commercial antioxidants used in boar semen liquid storage to mitigate the adverse repercussions of oxidative stress and enhance sperm quality within the storage period [5, 6]. For example, a dosage of 0.6 mg/mL isatis root polysaccharide in semen extender (an active compound found in Radix isatidis, the root of the Isatis tinctoria plant) improved the antioxidant capacity and boar sperm quality at 17°C for 3 days [6]. Similarly, Kou et al. [7] investigated the effect of icariin (the biologically active component of the herbal plant Epimedii herba) on preservation of boar spermatozoa at 17°C for 5 days, and evidenced that icariin exhibited antioxidant and antimicrobial properties which enhanced the sperm quality. Meanwhile, ashwagandha (Withania somnifera) belongs to the Solanaceae family and is generally referred to as Indian ginseng, poisonous gooseberry, or winter cherry [8]. Ashwagandha is enriched with diverse bioactive compounds, including alkaloids, amino acids, ergostane steroids, amino acids, and several neurotransmitters, showing broad therapeutic properties with the potential to prevent and treat various diseases directly or indirectly [9, 10]. In particular, ashwagandha root is rich in antioxidants and has anti-inflammatory properties, which are used to treat subfertility, increase sperm count, improve semen quality, regulate reproductive hormone levels, decrease oxidative stress, boost immunity, and improve cardiovascular health [8, 11]. Furthermore, Bhargavan et al. [12] reported that oral treatment of ashwagandha extract (AE) for 28 days, significantly increased the number of sperm, and motility, while reducing the ethanol-induced-oxidative damage leading to morphological abnormalities in the spermatozoa of adult male rats. Therefore, the purpose of this study is to investigate the sperm quality during the storage period in liquid boar semen containing different concentrations of AE, and suggests its potential application in assisted reproductive technology of animals.
MATERIALS AND METHODS
The dry powder of ashwagandha root (Classical Herbal Products, Kathmandu, Nepal) was mixed with distilled water (10 g powder in 100 mL water; final concentration 100 mg/mL) and boiled for 2 hrs at 100°C. The extract was filtered using a filter paper (Adventect®, Tokyo, Japan), and centrifuged at 180 × g for 5 min at 4°C. The supernatant was filtered using a 0.2 µm pore syringe filter (Corning®, Darmstadt, Germany), and stored at –80°C until use [13].
Liquid boar semen from mature Duroc boars (> 80% sperm motility) were purchased from a local AI center. Boar spermatozoa were washed, diluted with Beltsville thawing solution (BTS) [14] with AE (1, 5, 10, 20, and 50 mg/mL) or without AE (as a control), and then stored at 17°C for 5 days.
Sperm motility was measured using a computer-assisted sperm analysis (CASA) system (Sperm Class Analyzer®, Microptic S.L., Barcelona, Spain). Two microliter of spermatozoa was loaded into the chamber slide (Leja Products B.V., Nieuw-Vennep, Netherlands), and 10 optical fields (containing a minimum of 500 sperm cells per sample) were analyzed at 37°C. The percentages of total motile spermatozoa (%), progressive motile spermatozoa (%), hyperactive spermatozoa (%), and motion kinetic parameters were determined.
Spermatozoa were washed with PBS containing 0.1% polyvinyl alcohol (w/v; 0.1% PBS-PVA), and were stained using the LIVE/DEAD® Sperm Viability Kit (Molecular Probes, Eugene, OR, USA). The kit contains the DNA dyes SYBR14 (100 nM) and propidium iodide (PI, 10 μM) to differentiate between viable (SYBR14, green fluorescence, live) and non-viable (PI, red fluorescence, dead) spermatozoa (Fig. 1A). The images were acquired using a fluorescence microscope (Nikon Eclipse Ci microscope, Nikon Instruments, Tokyo, Japan), a camera (DS-Fi2, Nikon, Tokyo, Japan), and imaging software (version 4.30, Nikon).
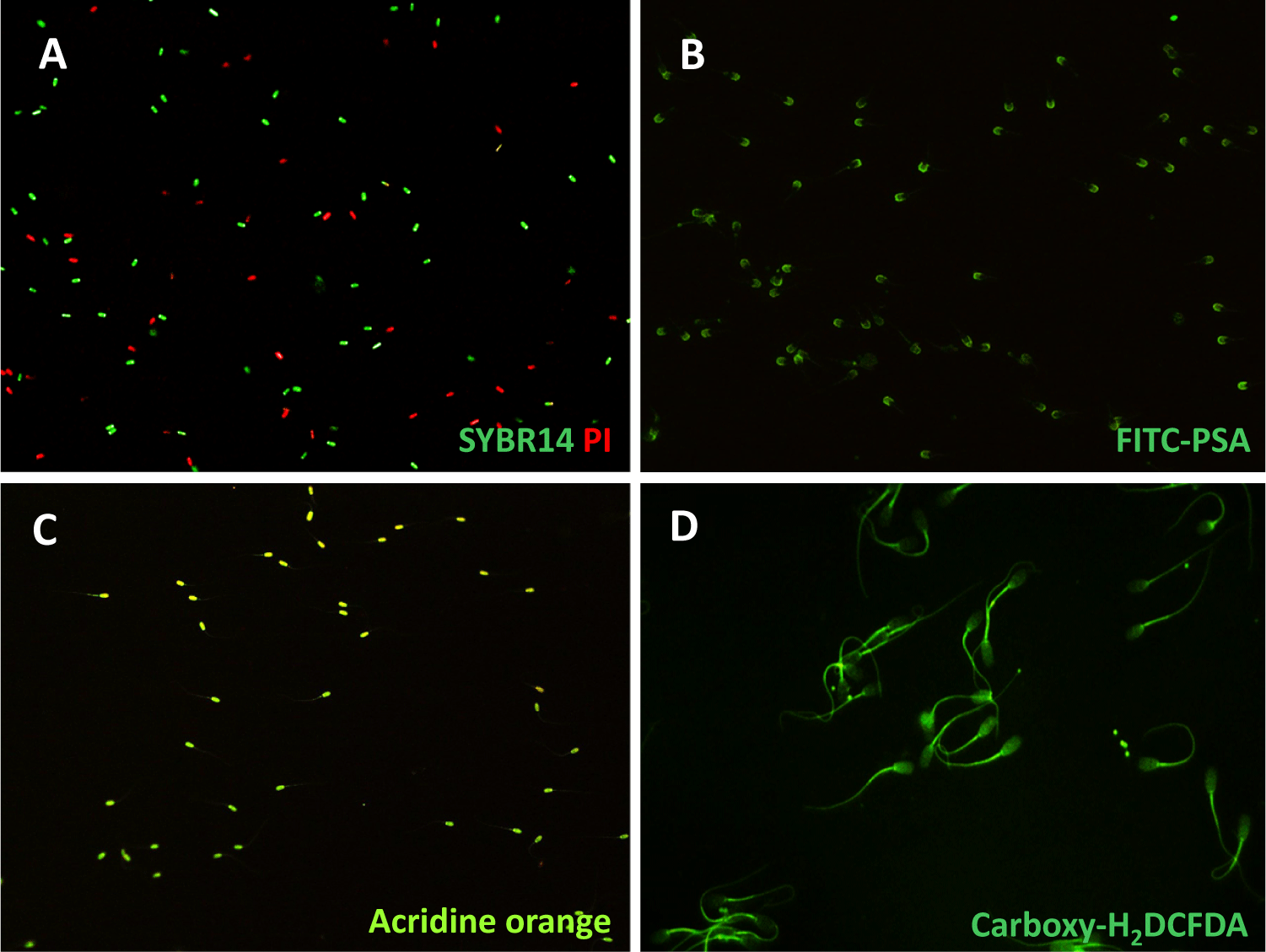
Boar spermatozoa were fixed in a 95% ethanol solution and then incubated at 4°C for 30 min. The sperm-fixed slides were air-dried and then stained with FITC-PSA (fluorescein isothiocyanate-Pisum sativum agglutinin) at 5 μg/mL for 10 min [15]. A fluorescence microscope and imaging software (Nikon Eclipse Ci microscope, Nikon Instruments) were used to determine acrosome integrity. The captured images were grouped into two categories based on the fluorescent signals detected in the sperm heads. The sperm heads showing green fluorescence were indicated as intact acrosome, while those with a lack of green fluorescence were counted as impaired or acrosome-reacted spermatozoa (Fig. 1B).
Sperm samples were prepared onto slides and allowed to air-dry after being fixed overnight in Carnoy’s solution (methanol:glacial acetic acid = 3:1) under chilled conditions. These fixed slides were incubated with a tampon solution (composed of 80 mM citric acid and 15 mM Na2HPO4, pH 2.5) at 75°C for 5 min. Following this, the slides were stained using the acridine orange solution (0.2 mg/mL). Then, the stain was removed with water, and coverslips were applied [16]. The slides were examined using a fluorescence microscope (Nikon Eclipse Ci microscope, Nikon Instruments). Normal sperm DNA displays by green fluorescence, while abnormal DNA emits yellow-green to-red fluorescence (Fig. 1C).
Spermatozoa were washed with 0.1% PBS-PVA, and incubated with 1 µM 5-(and-6) carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, Invitrogen, Eugene, OR, USA) at 37°C for 10 min. The stained spermatozoa were mounted on slide, and fluorescence intensity of reactive oxygen species (ROS) production (Fig. 1D) was measured under a fluorescence microscope (Nikon Eclipse Ci microscope, Nikon Instruments) [17].
Data were analyzed using one-way ANOVA on the GraphPad PRISM® (GraphPad Software, San Diego, CA, USA). A completely randomized design was applied, and Tukey’s multiple comparison test, and the t-test was used to compare the values of the individual treatments. Data were indicated as mean ± S.E.M., and statistical significance was denoted by p-values of *p<0.05, **p<0.01 and ***p<0.001.
RESULTS
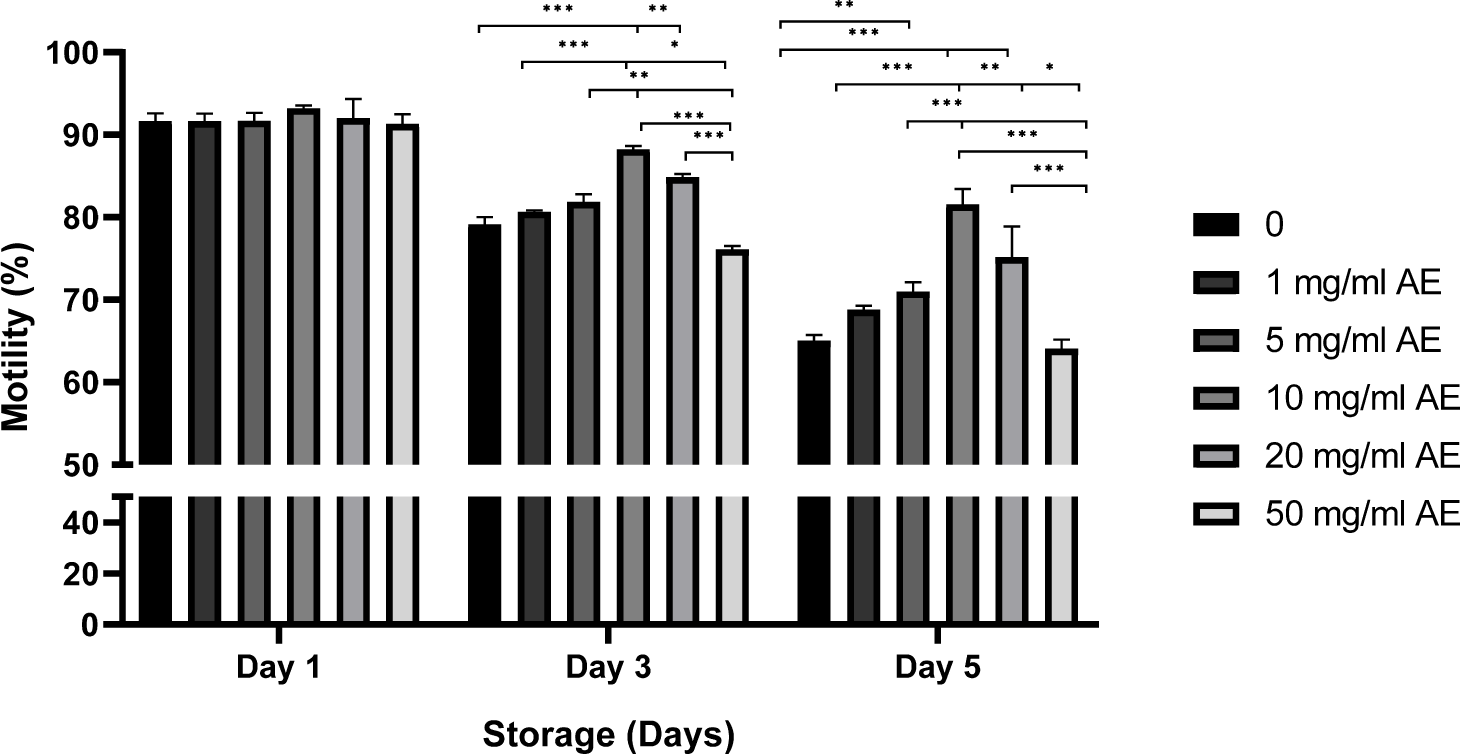
The motility of sperm stored in BTS with or without AE was examined during 5 days (Fig. 2). There was no significant difference in sperm motility between groups on day 1 of storage. A significant increase in sperm motility were observed in sperm stored in BTS with 10–20 mg/mL AE compared to control (without AE) or sperm stored with 50 mg/mL AE on day 3 (76.1%–79.1% in control and 50 mg/mL AE vs. 84.9%–88.3% in 10–20 mg/mL AE, p<0.001; Fig. 2). Higher sperm motilities were observed in sperm stored with 1–20 mg/mL AE on day 5 (64.1%–65.0% in control and 50 mg/mL AE vs. 68.8%–75.2% in 1–20 mg/mL AE, p<0.01, p<0.001; Fig. 2). In motion kinetic parameters, the percentages of sperm progressive motility, linearity index, straightness index, curvilinear velocity, straight line velocity, oscillation index and average path velocity increased in spermatozoa stored in BTS containing AE compared to the control (p<0.01, and p<0.001; Table 1).
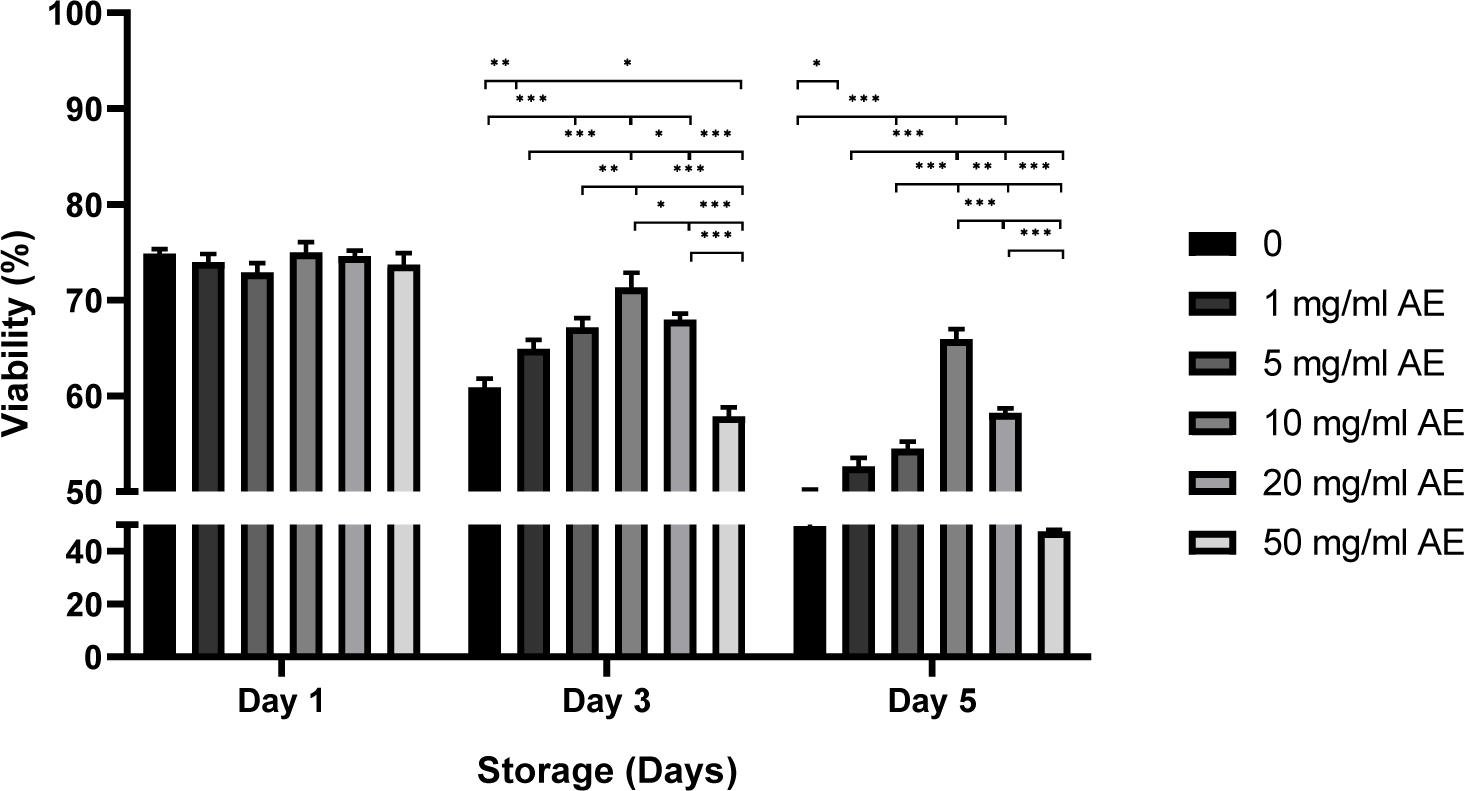
Sperm viability also showed no difference between groups on day 1 (Fig. 3). When spermatozoa were stored in BTS with 1–20 mg/mL AE for 3 days, the viability increased significantly compared to control (without AE) or sperm stored with 50 mg/mL AE (57.9%–60.9% in control and 50 mg/mL AE vs. 64.9%–68.0% in 1–20 mg/mL AE, p<0.01, p<0.001; Fig. 3). Statistically higher viability was obtained in sperm stored in 10–20 mg/mL AE compared to the control and 50 mg/mL AE on day 5 (47.6%–49.5% in control and 50 mg/mL AE vs. 58.2%–65.9% in 10–20 mg/mL AE, p<0.001; Fig. 3).
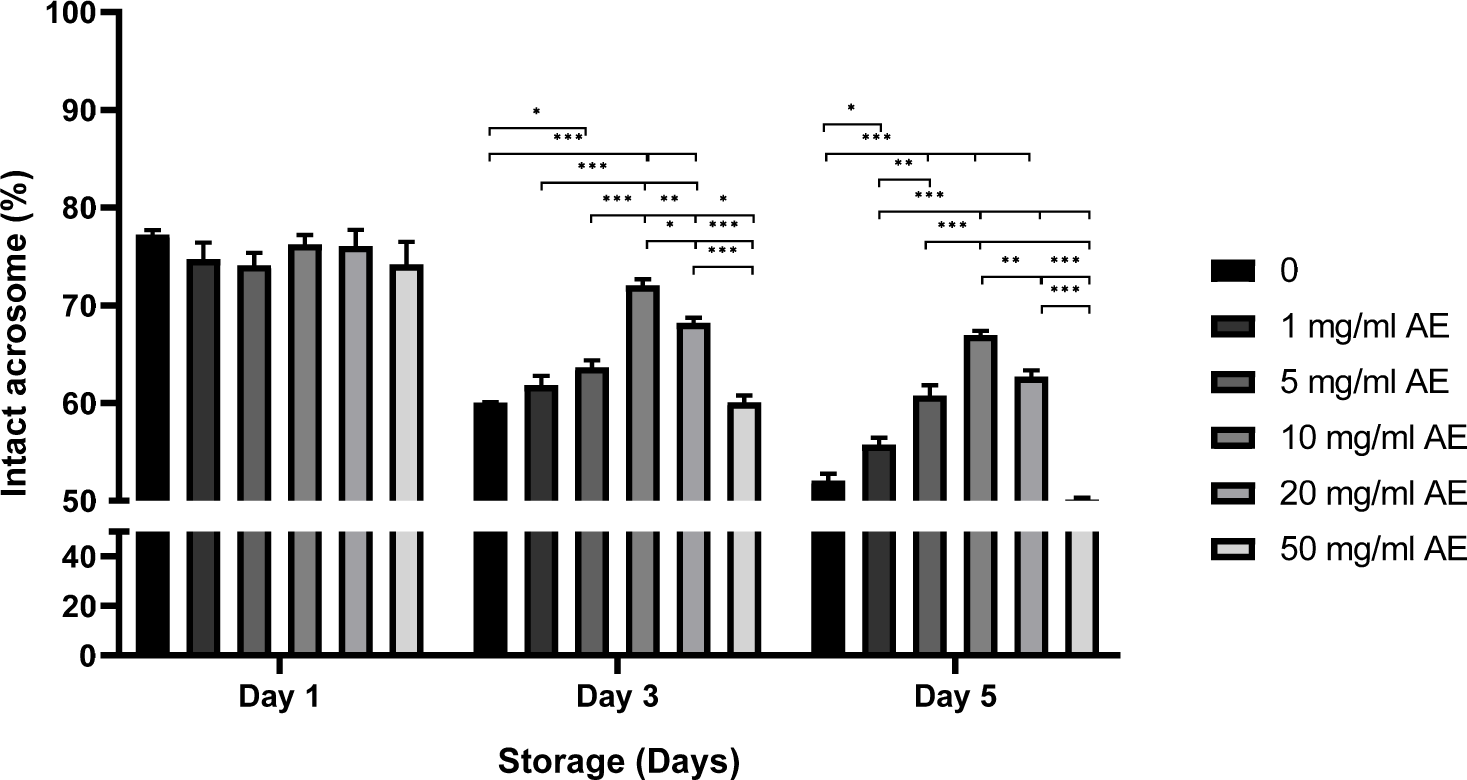
There was no statistical difference in the rate of intact acrosome on day 1 (Fig. 4). Significantly higher rates of intact acrosome were seen in sperm stored with 5–20 mg/mL AE (60.0%–60.1% in control and 50 mg/mL AE vs. 63.7%–68.2% in 5–20 mg/mL AE, p<0.05, p<0.001; Fig. 4) on day 3 of storage. Additionally, the rates of intact acrosome were higher in sperm treated with 1–20 mg/mL of AE on day 5 (50.1%–52.1% in control and 50 mg/mL AE vs. 55.7%–62.7% in 1–20 mg/mL AE, p<0.05, p<0.001; Fig. 4).
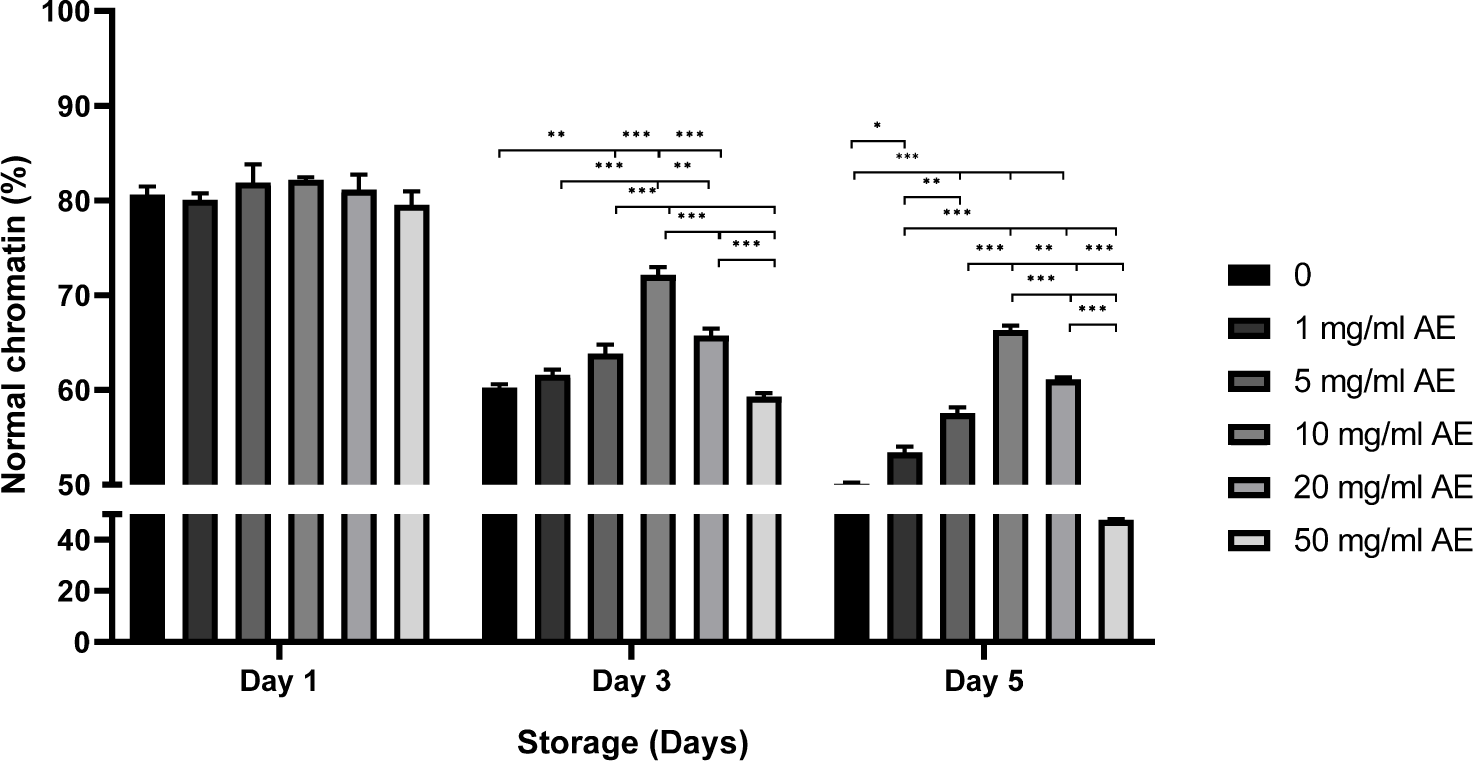
Although, there is no difference in the rate of normal chromatin on day 1, a higher rate of normal chromatin was obtained in sperm treated with 10 mg/mL AE on day 3 (72.1%) than those of other treatments (59.3%–61.6%; Fig. 5). Similar tendencies were observed on day 5 in sperm stored in 1–20 mg/mL AE (47.8%–50.1% in control and 50 mg/mL AE vs. 53.4%–61.1% in 1–20 mg/mL AE, p<0.05, p<0.001; Fig. 5).
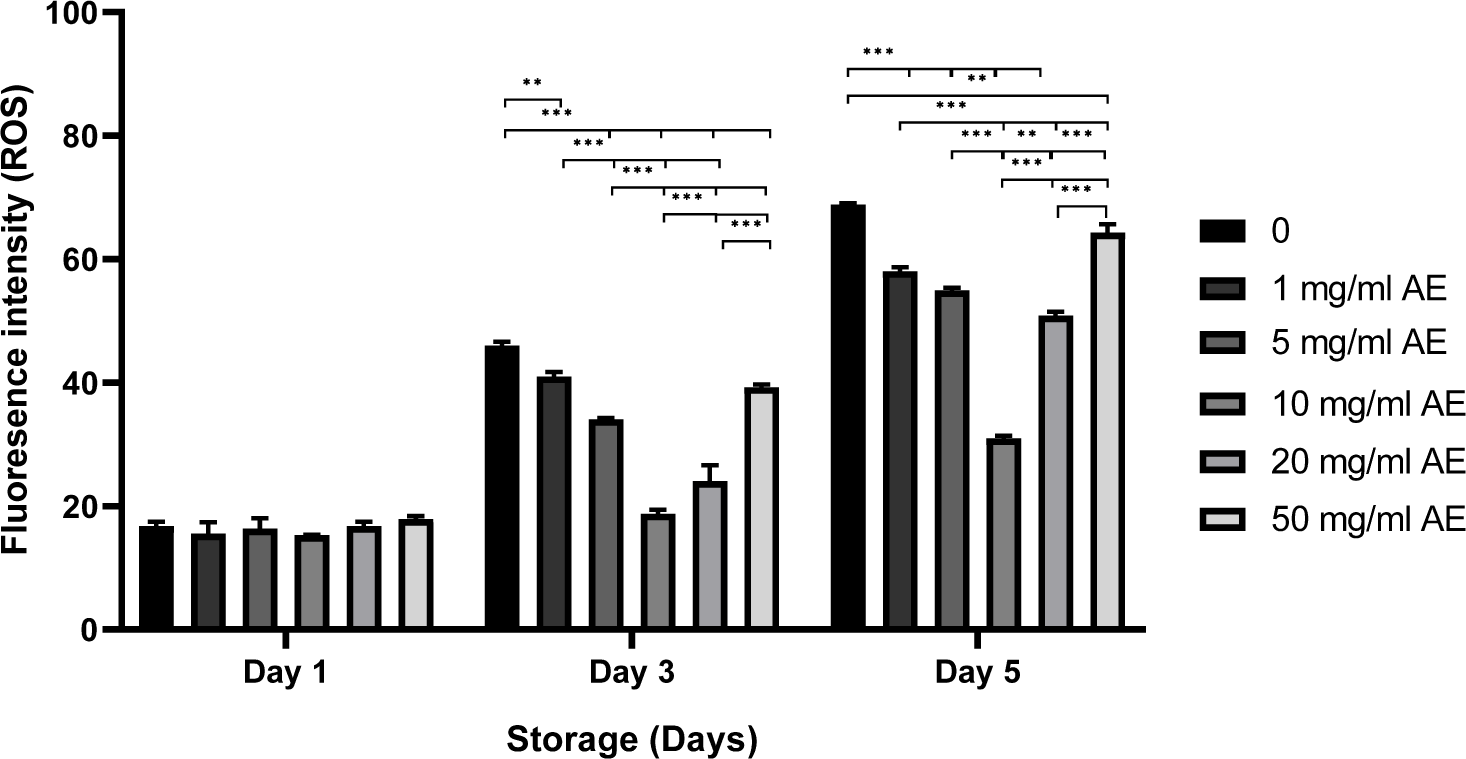
The fluorescence intensity of ROS level was measured after spermatozoa staining with carboxy-H2DCFDA; Fig. 6. Compared to the control group stored in BTS without AE, the sperm treated with 1–20 mg/mL AE showed lower fluorescence intensity on day 5 (p<0.01, p<0.001; Fig. 6). The lowest fluorescence intensity of ROS production was obtained in sperm stored in BTS with 10 mg/mL AE during 3–5 days.
DISCUSSION
This study investigated the effects of AE as an additive with antioxidant properties to enhance sperm quality in boar semen stored at 17°C. As expected, AE at concentrations of 1–20 mg/mL in liquid boar semen, maintained semen quality during storage, resulting in higher values of motility, viability, acrosome integrity, and chromatin stability in boar spermatozoa than the control group stored without AE. Oxidative damage following in vitro storage has been identified as one of the major contributors to sperm failure. Hence, identifying an optimum antioxidant is crucial for preserving sperm quality [18, 19]. AE has been shown to increase antioxidant activity and reduce ROS levels by increasing the activity of superoxide dismutase and catalase enzymes in animal models [20–22]. The compounds found in AE can donate electrons and interrupt the harmful chain reaction of free radical production, thereby reducing the overall burden of ROS [23].
Sperm motility is a direct indicator of sperm quality, and is also an important predictor for successful fertilization [24]. Ren et al. [6] reported that polysaccharides extracted from isatis roots have excellent antioxidant effects, and its addition protected boar sperm from oxidative stress and maintained higher sperm motility during the storage. Similarly, the results of the present study indicated that sperm stored in the presence of 1–20 mg/mL AE displayed higher motility than the control group (Fig. 2). In contrast, previous studies have shown that the addition of resveratrol (a natural antioxidant found in various plant species), known to have antioxidant properties, to boar semen, had no effect sperm motility [25, 26].
The sperm plasma membrane regulates the homeostasis between the sperm's internal and extracellular environment and facilitates sperm capacitation and the acrosome reaction [24]. Previous studies have indicated that the plasma membrane of boar sperm contains polyunsaturated fatty acids that are sensitive to lipid peroxidation damage caused by ROS, thereby lowering sperm motility and viability [27, 28]. In the present study, sperm viability was significantly higher on days 3 and 5 of storage at concentrations of 1–20 mg/mL AE compared to the control (Fig. 3). Acrosome integrity and chromatin stability can be supported with other references of similar studies in this section.
In conclusion, our study highlights the role of AE, as an antioxidant, as it positively enhances sperm motility, and reduces intracellular ROS production during liquid boar semen storage at 17°C, at optimal concentrations of 10 mg/mL AE in BTS. These findings suggest that AE has potential as a supplement in boar semen storage and assisted reproductive technology during animal fertility treatments.







