INTRODUCTION
Carrageenan (CGN) is widely approved and utilized as an additive in various domains, including food, fragrance, and cosmetics, owing to its properties of gelling, thickening, and stabilizing [1]. Conversely, CGN is frequently employed as an agent inducing inflammation (such as paw edema, air pouch, ulcerative colitis, peritonitis, etc.) in animal experimentation [2–5]. It is commonly acknowledged that adjuvants operate by establishing an antigen depot effect at the injection site and stimulating innate immune responses [6, 7]. The viscosity and inflammation-inducing nature of CGN may confer characteristics of an immunological adjuvant.
Previous report have shown that the potential of CGN as an adjuvant for subcutaneous immunization [8]. However, scant research has addressed the immune response of the esophagus [9]. Despite numerous studies focusing on oral tolerance (the specific suppression of immune responses to food antigens) in the gut, few have delved into the esophageal context [10].
Initially, this study aimed to assess the impact of CGN as an adjuvant on oral immunization. However, the preliminary experiments using oral gavage yielded significant deviations. Upon investigating this issue, it was discerned that varying results ensued based on the depth of insertion of the oral gavage needle.
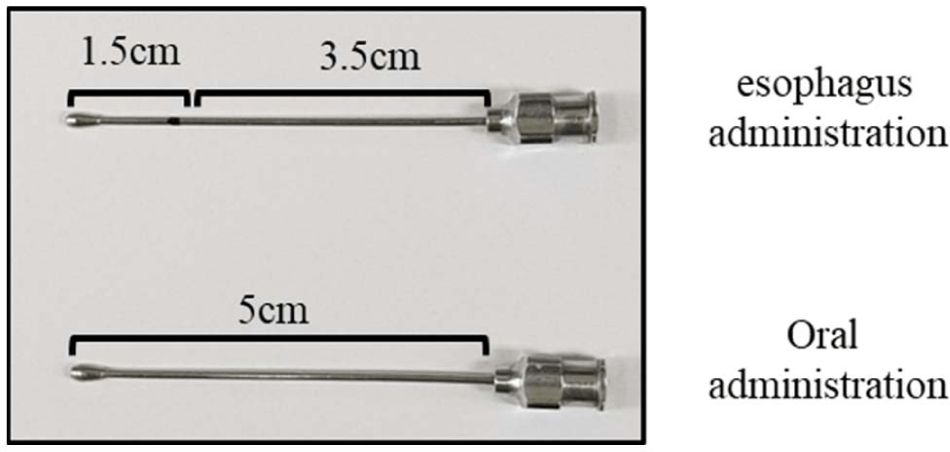
In this study, the introduction of the antigen through the insertion of a needle with a total length of 5 cm into the mouth was defined as oral immunization, while shallow injection up to approximately 1.5 cm was termed esophageal immunization. Consequently, the study׳s objective was refined to examine the influence of CGN as an adjuvant on both oral and esophageal immunity.
MATERIALS AND METHODS
Female ICR (CD-1) mice, aged 8 weeks, were utilized in this study. They were provided with water and food ad libitum, with seven to eight mice per group. The antigen employed for immunization was bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA). For both oral and esophageal immunity, BSA antigen was prepared at a concentration of 100 μg/mouse, while the adjuvant CGN (Lambda CGN) was prepared at a concentration of 500 μg/mouse. These were dissolved in 200 μL of DPBS per animal and injected accordingly. Following immunization, drinking was restricted for one hour to prolong the antigen retention in the esophagus for a designated period. Immunization was attempted either once or twice at two-week intervals. Subsequently, blood samples were collected from the tail vein, and the serum was separated from the blood. Antibody titer was evaluated at a 1:100 serum dilution using enzyme-linked immunosorbent assay (ELISA).
All HRP-conjugated secondary antibodies targeting mouse immunoglobulins (total IgG, IgG1, IgG2a, IgA) for ELISA were procured from Sigma-Aldrich. In this study, oral immunization involved inserting a 5 cm long oral gavage needle through the mouth and administering the antigen. Esophageal immunization was conducted similarly, albeit with only 1.5 cm of the needle being inserted (Fig. 1).
All animal experiments were conducted in compliance with guidelines set forth by the Care and Use of Research Animals and were approved by the Animal Studies Committee of Dankook University (approval number: DKU-20-048). Statistical analyses were performed using GraphPad Prism 8.01 (GraphPad, San Diego, CA, USA). Significant differences among groups were assessed using one-way ANOVA analysis of variance. Results are presented as the mean ± S.E.M., and p-values are presented.
RESULTS AND DISCUSSION
BSA or BSA + CGN was administered either to the stomach (oral immunization) or the upper esophagus (esophageal immunization) using a gavage needle. Subsequently, blood samples were collected from the tail vein two weeks later, and the serum antibody titer (total IgG) was assessed (Fig. 2). The findings revealed that in cases of oral immunization, antibodies were undetectable irrespective of the presence or absence of CGN as an adjuvant. Conversely, following esophageal immunization without adjuvant, low antibody titers were detected in only a minority of mice. However, when administered into the esophagus alongside CGN as an adjuvant, a substantial number of mice exhibited elevated antibody levels. Nevertheless, within the same group of mice, antibody formation was absent in some individuals. It's noteworthy that the esophageal immunization employed in this study represents an experimental approach that has not been previously reported and was first attempted herein.
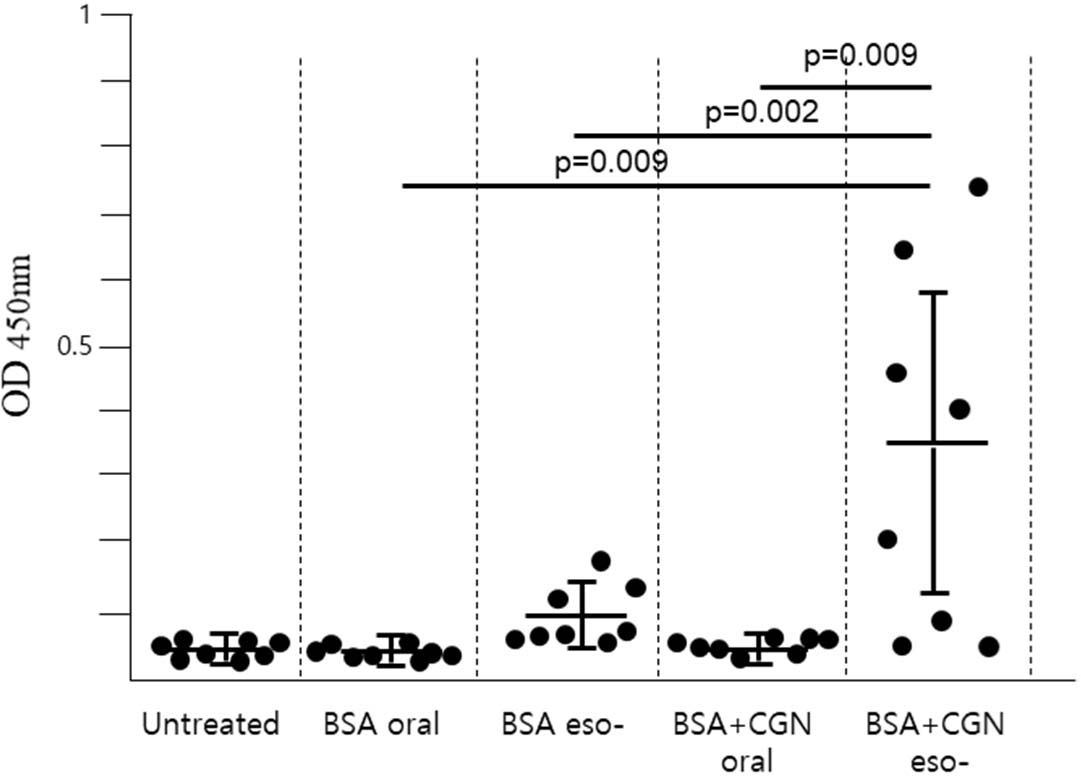
To ensure that the antigen adequately stimulated the esophageal mucosa, it was shallowly injected into the upper esophagus using an oral gavage needle, and drinking water was withheld for one hour post-injection to prolong the antigen׳s residence time in the esophagus. Despite these measures, as evidenced by the results, a subset of mice failed to generate antibodies altogether. This suggests that additional, more refined experimental conditions may be necessary beyond those considered for esophageal immunity in this study.
The esophagus serves as the conduit for transporting food and liquids from the mouth to the stomach. It is well-documented that the normal transit time of the esophagus is approximately 10 s [11]. Considering this, it is hypothesized that for an immune response to occur in the esophagus, it may be necessary for the antigen to linger in the esophageal mucosa for an extended duration. Thus, one of the effects of CGN as an esophageal immune adjuvant could be the prolongation of antigen residence time in the esophageal mucosa, given the viscosity of CGN.
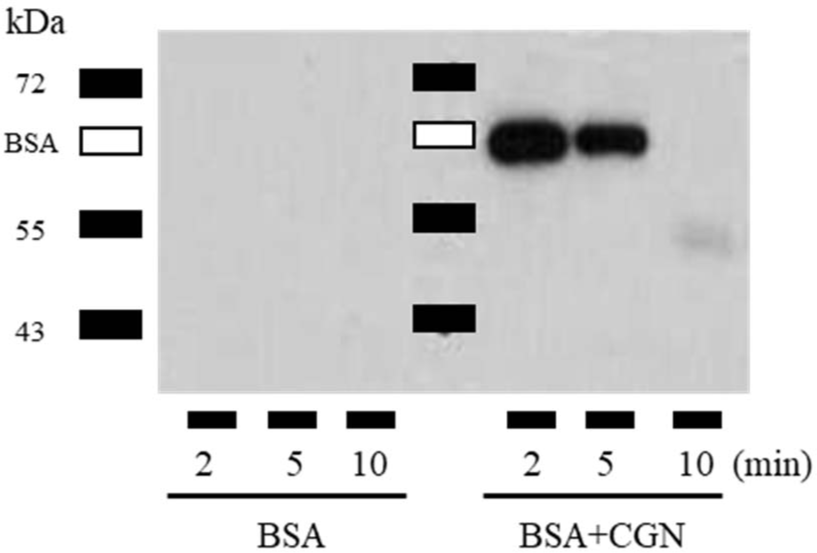
To investigate this, BSA and BSA + CGN were administered to the esophagus in a manner analogous to mouse immunization. Subsequently, the esophagus was dissected at 2, 5, and 10-minute intervals. These esophagi were then perfused with 100 μL DPBS containing 0.1% Tween-20 and a protease inhibitor cocktail. The presence of the antigen (BSA) in the perfusate was confirmed via western blot. This experiment was independently conducted three times, and a representative result is presented. The results indicated that while BSA alone resulted in no detection of BSA in the esophagus at 2 min, co-administration with CGN led to a significant presence of BSA even after 2 to 5 min (Fig. 3). Thus, these results confirm that the co-injection of antigen and CGN extends the antigen's retention time in the esophagus.
Since the esophagus acts as a passage for foreign substances to enter the stomach, it is highly likely that the body will perceive it as a foreign substance entering the body. Therefore, the esophagus is considered an organ of immune tolerance. Consequently, the esophagus is believed to be an organ of immune tolerance. The rapid transit of food mentioned previously may contribute to the lack of immune response in the esophagus. Consequently, CGN not only prolongs antigen residence time in the esophageal mucosa but also exerts an inflammatory effect. In considering this scenario as a potential trigger for inducing an immune response in the esophagus, it cannot be discounted that the consumption of viscous and inflammatory foods may unexpectedly induce an immune response in the esophagus.
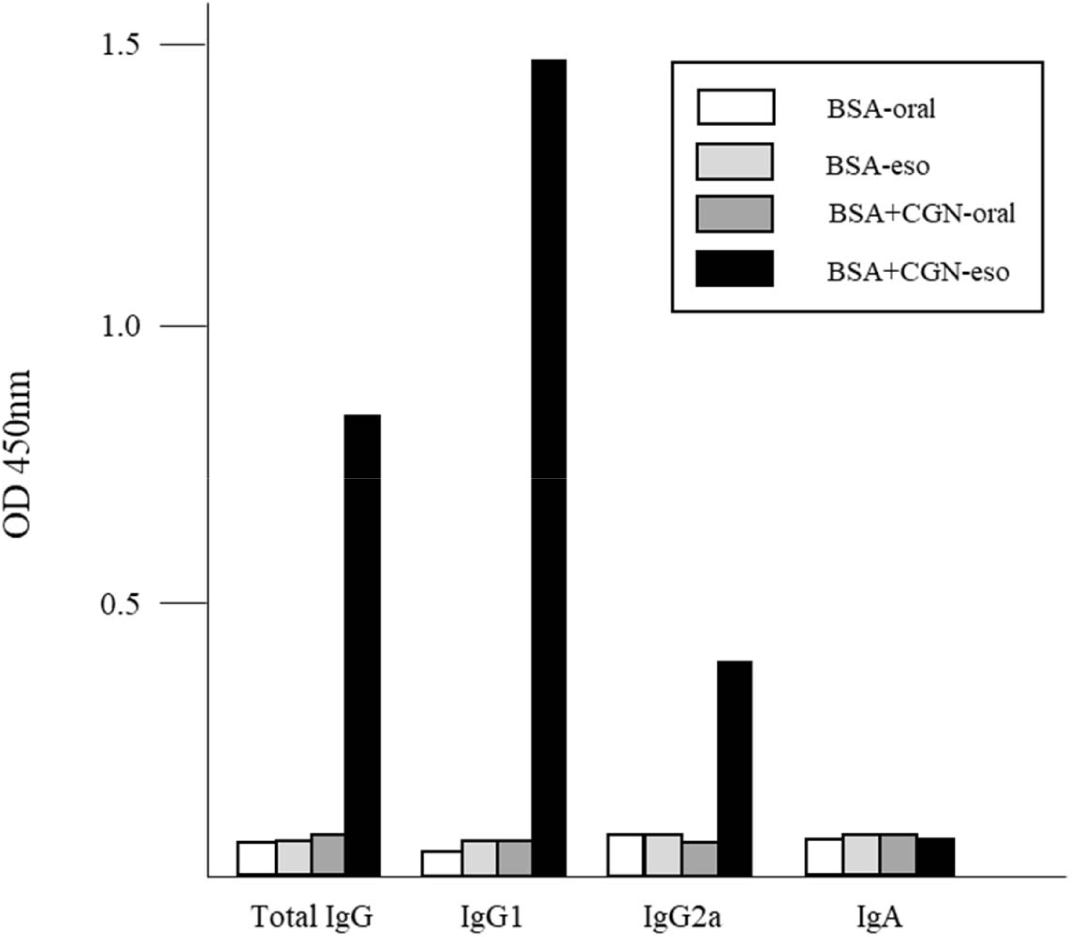
As the esophagus comprises mucosal tissue [12], we investigated whether esophageal immunization triggered mucosal immunity. Immunizations were conducted twice at 2-week intervals. Four weeks after the initial immunization, blood samples were collected from the tail vein, and serum antibody titers (total IgG, IgG1, IgG2a, and IgA) were assessed.
Results indicated that none of the four antibody types examined were detected following oral administration or esophageal administration without CGN adjuvant. Conversely, the production of total IgG, IgG1, and IgG2a was observed solely in mice immunized via the esophagus with both antigen and adjuvant. However, the presence of IgA, a hallmark of mucosal immunity [13], was not detected in serum (Fig. 4). While IgA production, a representative of mucosal immunity, could not be confirmed in serum, the potential presence in tissues remains plausible. Although numerous studies have explored immune responses in mucosal tissues, including the gastrointestinal mucosa [14, 15], research on esophageal mucosal immunity is relatively scarce. Hence, further investigations into esophageal mucosal immune responses are warranted.
In conclusion, this study verified the production of IgG antibodies against BSA when the antigen (BSA) was injected into the esophagus with CGN adjuvant. Research on esophageal immunity has been scarce in the literature.
This study suggests the possibility of esophageal immunity under the two conditions of utilizing CGN as an adjuvant and shallow insertion of an oral gavage needle. However, notable discrepancies in results, such as the absence of antibody production in some mice within the same group, underscore the need for more precise experimental conditions and further research.







