INTRODUCTION
Meningoencephalomyelitis of unknown etiology (MUE) is an inclusive term for non-infectious inflammatory diseases of the central nervous system (CNS). It has several subtypes comprising granulomatous meningoencephalomyelitis (GME), necrotizing meningoencephalitis, and necrotizing leukoencephalitis [1]. The MUE is usually diagnosed tentatively based on the manifestation of neurologic signs, abnormal findings on magnetic resonance imaging (MRI), and cerebrospinal fluid (CSF) analysis. A histopathological examination is required for a definitive diagnosis [2]. The clinical signs of MUE are associated with affected parts of the CNS and are variable, including ataxia, seizure, altered mentation, nystagmus, and blindness [1, 2].
Despite the exact pathogenesis of MUE remaining unknown, immunosuppressive therapy is the cornerstone of the treatment [1]. As with other immune-mediated diseases in small animals, glucocorticoids (GCs) are the mainstay of the therapy. However, secondary agents such as mycophenolate mofetil (MMF), cyclosporine (CsA), and cytosine arabinoside (CA) could be used [3–6]. The prognosis of MUE is generally known as poor to life-threatening, but the prognosis might be variable with the appropriate treatment [1, 4–6]. Several studies reveal better overall outcomes of administering the combination of immunosuppressive agents with GC compared with administering GC alone [4–6].
We describe a clinical course of a dog with GME who was refractory to treatment, and factors related with the resistance to conventional immunosuppressive therapy.
CASE REPORT
A one-year-old female Maltese dog was presented at a local animal hospital with left-sided head tilting, horizontal nystagmus (fast phase to the right), and tetraparesis. Blindness was the first clinical sign and was relieved after administering prednisolone (0.5 mg/kg, PO, q 12 h; Solondo, Yuhan Pharma, Seoul, Korea). However, about three weeks later from the onset of blindness, the patient showed additional neurological symptoms, including left-sided head tilt, leaning, and falling with horizontal nystagmus. The MRI was performed, and it revealed inflammatory lesions on the cerebellum and brainstem. The immunosuppression therapy was started based on the clinical signs and MRI findings. Methylprednisolone sodium succinate (30 mg/kg, IV, for 30 min; Predisol, Reyonpharm, Seoul, Korea), MMF (10 mg/kg, PO, q 12 h; Myconol Cap., Hanmi Pharm, Seoul, Korea), and CA (50 mg/m2, SC, q 12 h for 2 days, repeated q 3 wk; Cytosar-U Ing., Pfizer, New York, NY, USA) were prescribed. However, the patient’s neurological signs gradually progressed.
At the presentation, there were left-sided head tilt with right jerk horizontal nystagmus and tetraparesis on neurological examination. The conscious proprioception was decreased to absent in the four limbs. The MRI was performed again on the 74th day from the first MRI scanning, revealing diffuse lesions on the brainstem and cerebellum (Fig. 1). Compared to the previous MRI results, the progression of the inflammatory lesions was confirmed. In the CSF analysis, the total nucleated cell count (TNCC) was 85 cells/uL (reference range, < 5 cells/uL), and the cytological examination of CSF revealed a moderate to marked mononuclear pleocytosis. Bacterial culture and polymerase chain reaction for infectious agents were performed (IDEXX Laboratories, Westbrook, ME, USA) to rule out infections, and there were no specific findings on both tests results.
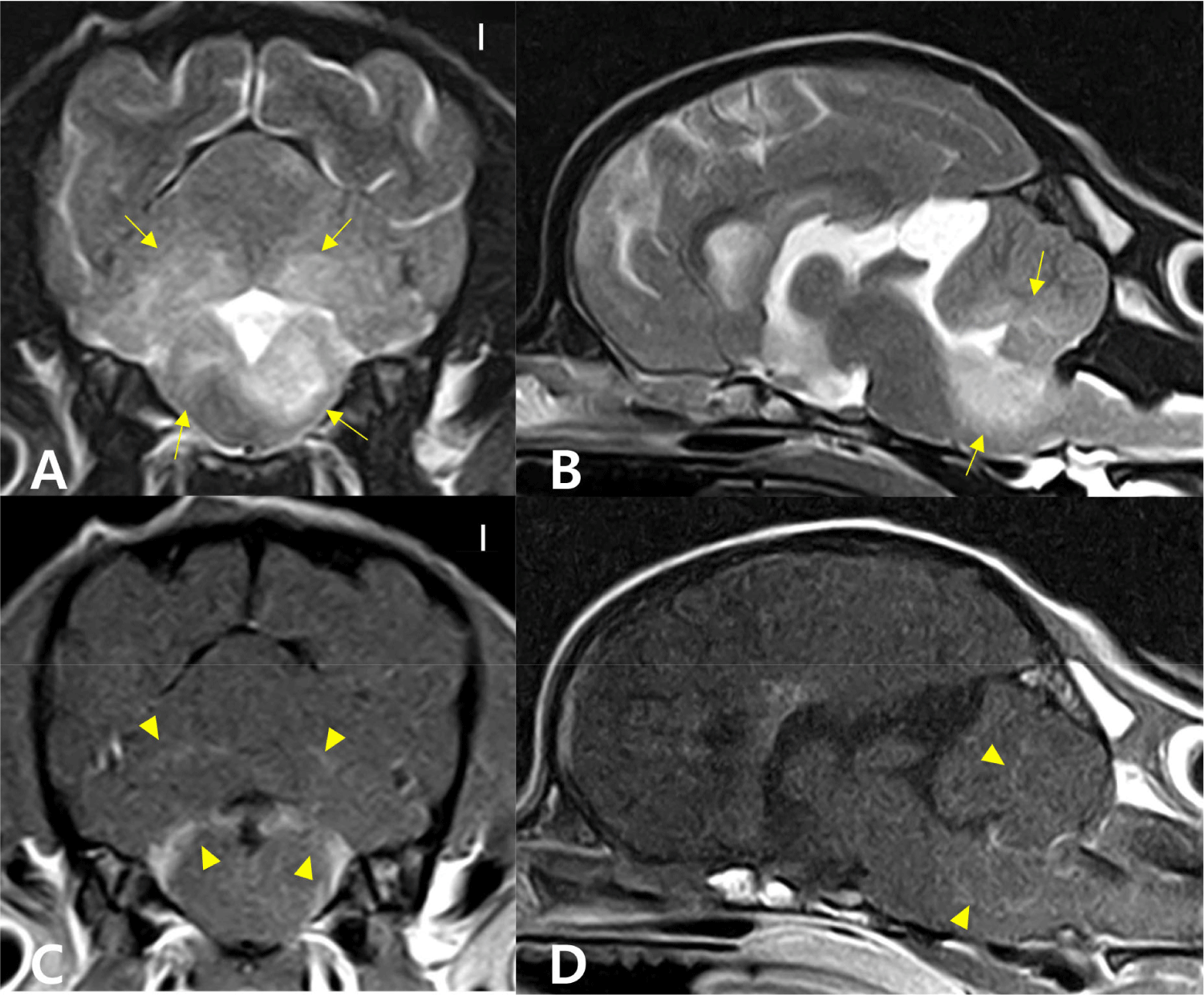
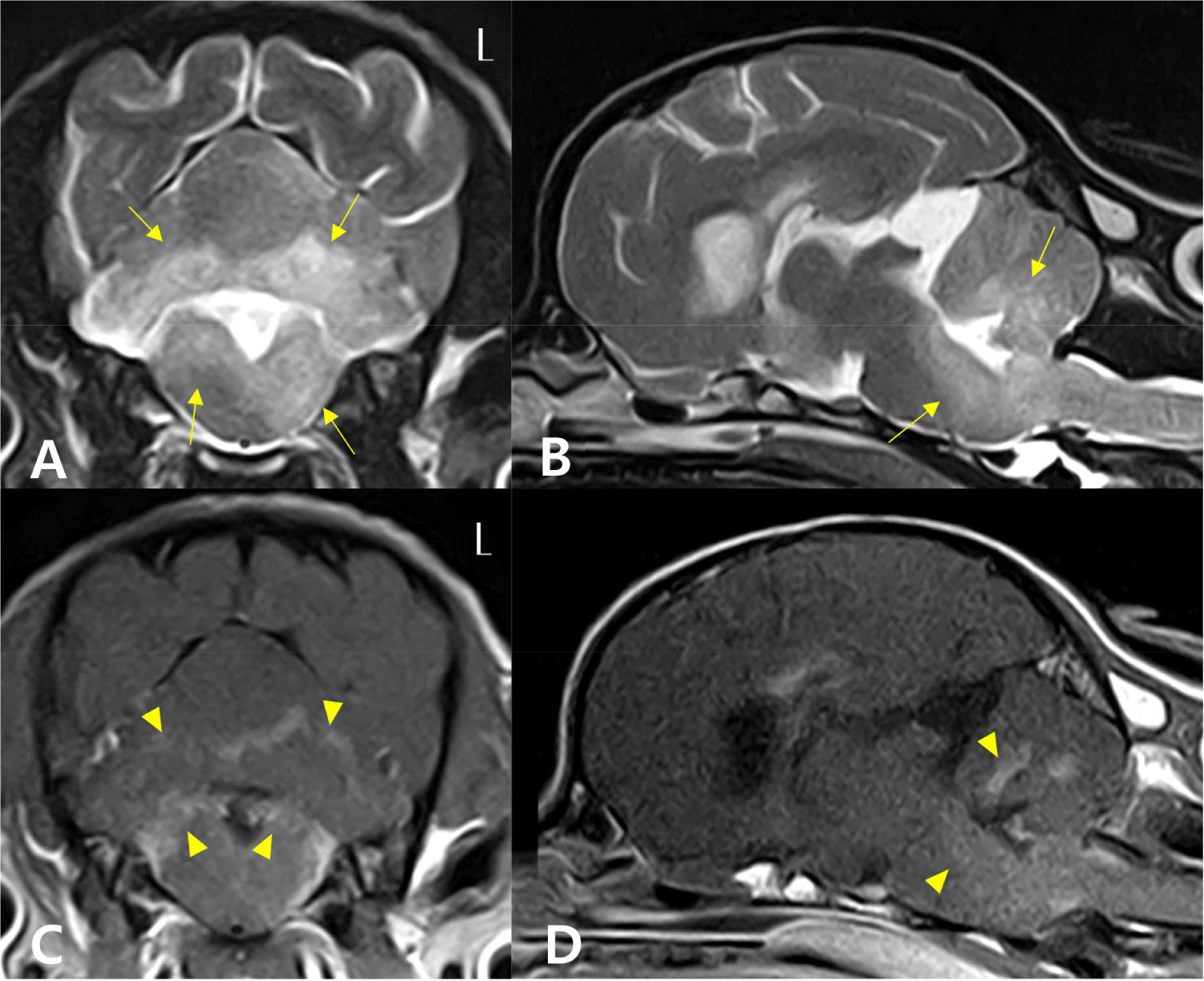
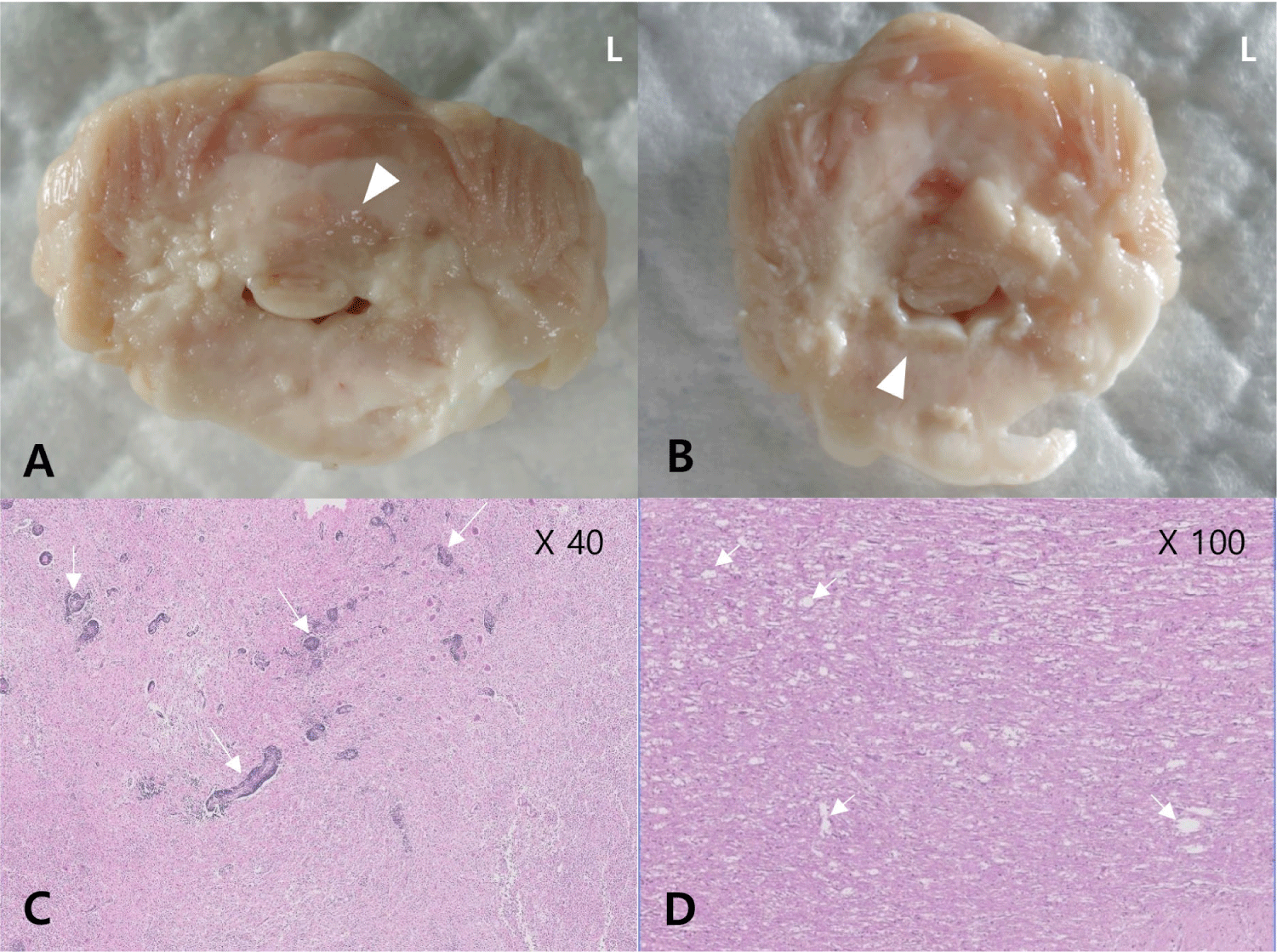
Based on the MRI findings and CSF analysis results, we tentatively diagnosed the patient with MUE and started the multiple immunosuppressive therapy with CA (300 mg/m2, IV for 30 h, repeated q 3 wk), leflunomide (2 mg/kg, PO, q 24 h; Avara Tab., Sanofi, Paris, France), and prednisolone (1.5 mg/kg, PO, q 12 h). We shifted the type of immunosuppressive agents with maintaining the number of drugs considering the poor response to medications which had been prescribed before. There was no improvement in neurological symptoms, and the CsA (25 mg/kg, PO, q 24 h; Cipol-N Soft Cap., Chong Kun Dang, Seoul, Korea) was added after 3 days of the initiation of the treatment. On the 7th day, the nystagmus disappeared, and the conscious proprioception normalized in all four limbs. However, the neurological signs were progressive, the owner decided to perform euthanasia 108 days after the initiation of the treatment. The brain MRI scan and CSF analysis were repeated. The progression of lesions was confirmed compared with the previous MRI results (Fig. 2). In the repeated CSF analysis, marked mononuclear pleocytosis (160 cells/uL of TNCC) was observed. A necropsy was performed. The macroscopic examination of the brain sections showed inflammatory lesions over the brainstem and cerebellum (Fig. 3A and B). On the histopathological examination, there was an intense inflammatory zone comprising infiltrates of epithelioid macrophages, lymphocytes and plasma cells on the cerebellum and the spinal cord, with inflammatory infiltrates extending to the overlying meninges (Fig. 3C and D). The patient was definitively diagnosed with disseminated GME involving the brainstem, cerebellum, and spinal cord.
DISCUSSION
GME is an idiopathic CNS inflammation that can progress rapidly in dogs [1, 2]. The pathogenesis of canine GME remains unclear, but the T cell-mediated autoimmune response was suggested [1, 7]. GC monotherapy may be acceptable, but the outcomes are variable, and adverse effects are common in dogs with a high dose of GC including polyuria, infections and iatrogenic hyperadrenocorticism [1, 3]. The patient showed poor response to triple immunosuppressive therapy. Thus, we used four different immunosuppressive drugs: prednisolone, CsA, leflunomide, and CA. The immunosuppressive drugs those we used have different mechanisms but ultimately suppress T cell activation [5, 8]. However, the patient showed little clinical improvement and the lesions of the brain had worsened. Based on the treatment responses of this case, we concluded that other pathological and drug-resistance (DR) mechanisms could be involved in the inflammatory response of the CNS with GME.
The DR can be present by intrinsic and acquired. Mechanisms of DR include impaired drug delivery or cellular uptake, and changes in drug target levels [9]. The P-glycoprotein (P-gp), encoded by the MDR1 gene, is a cellular membrane protein which plays a role in DR via the efflux capacity [9]. The overexpression of P-gp on canine lymphocyte was found in patients with multidrug resistance [9], and increased expression of P-gp on T cells in human patients with autoimmune disease who are less responsive to therapy [10]. The CsA is a P-gp inhibitor which reduces expression or function of P-gp [10]. In this case, after administration of CsA, the patient showed transient improvements of neurological symptoms. Therefore, it might be necessary to investigate the DR related factors in patients not responding well to conventional immunosuppressive therapy. In addition, further research for treatment that can regulate DR factors is required.
In MUE patients who are refractory to the conventional therapy, treatments which targets different mechanisms compared to immunosuppressants could be considered. Tyrosine kinase inhibitors (TKIs) is a stand out candidate. Recent studies on canine MUE found the expression of several tyrosine kinases on inflammatory lesions of brain tissue [11], and one study using TKIs in MUE cases showed favored outcomes [12]. Therefore, further study on treatment of GME which acts on these different mechanisms is needed for patients who respond poorly to conventional immunosuppressive treatment.
The presence of multifocal neurologic signs or focal signs with involvement of the brainstem region and a high TNCC at the time of diagnosis are known as poor prognostic factors [4, 5]. In this case study, the patient showed multifocal neurologic signs including brainstem signs and high TNCC (after 2 months of treatment) on CSF analysis. In this patient, the acute onset of blindness could have been the first neurological symptom. Although the prednisolone was immediately prescribed after the onset of vision loss, the patient presented with additional neurological symptoms after three weeks and combination immunosuppressive therapy was initiated. It is known that the timing of initiating the immunosuppressive therapy is critical, and it can affect the survival time. Thus, rapid administering multiple immunosuppressive agents might be an important part of the treatment of canine GME. In addition, the ocular form of GME can progress to disseminated CNS lesion [2]. Therefore, in canine GME, when the patient is identified as having negative prognostic factors, anticipating that the patient might be refractory to treatments such as DR or rapid progression of disease, different drug consideration might be needed.
In canine MUE, it is known that the timing of the treatment is important. If patients with GME have poor prognostic factors, more aggressive and immediate treatment might be necessary. Despite the pathogenesis of GME as T cell-mediated inflammation, other mechanisms might also be at play. These mechanisms could respond poorly to conventional treatment. Further studies are required to understand the exact mechanisms of inflammation of GME and to conduct a new treatment modality.







