The non-ionic surfactant, 2-{2-[2-(4-nonylphenoxy)ethoxy]ethoxy}ethan-1-ol (CAS No. 127087-87-0), is used as a raw material for herbicides, cosmetics, paints, plastics, disinfectants, and detergents [1-3]. It has been widely used as an active ingredient in various spermicides for decades [4]. However, few studies elucidate on its toxicity, revealing effects that are non-specific. In the cosmetic industry, it is used as an emulsifier, moisturizer, and solubilizer, as well as in hair and skin care products, baths, and shaving products [3]. It is often found in products that can be inhaled, thereby being hazardous to workers through inhalation. However, there have been no studies on the risk of inhalation exposure to 2-{2-[2-(4-nonylphenoxy)ethoxy]ethoxy}ethan-1-ols.
Therefore, to prevent health disorders in workers who may be exposed through the respiratory tract of 2-{2-[2-(4-nonylphenoxy)ethoxy]ethoxy}ethan-1-ol at industrial sites, toxicity was evaluated to serve as a basis for its classification as a hazardous chemical substance [5]. To investigate the acute inhalation toxicity of the test substance, the rats were exposed to the test substance at concentrations of 0.5, 1, and 5 mg/L for 4 hr. Subsequent clinical symptoms and weight changes were observed, and an autopsy was performed after 14 days to observe the gross lesions and evaluate the toxicity of each major organ.
We purchased 7-week-old specific pathogen-free Sprague-Dawley rats from Central Lab Animal (Seoul, Korea), acclimatized them for 2 weeks, and exposed to the test substance using the mist generation and monitoring test equipment in the nose-only inhalation chamber. Three males and three females were exposed per concentration.
Also, 2-{2-[2-(4-nonylphenoxy)ethoxy]ethoxy}ethan-1-ol was purchased from Sigma Aldrich (Burlington, MA, USA). It was sprayed with a mist generator (HCT, Incheon, Korea), mixed with clean air, and supplied to the suction chamber at a selected concentration. Its exposure concentration was determined according to the Organisation for Economic Co-operation and Development (OECD) Guideline for the Testing of Chemicals Section 4 Health Effects Test No. 436 Acute Inhalation Toxicity – Acute Toxic Class Method – Annex 3d [6, 7]. Due to the rarity of information on its inhalation toxicity, 5 mg/L was considered the starting concentration (maximum), and exposure concentrations were tapered to 1 mg/L and 0.5 mg/L, each for 4 hours.
The actual concentration was calculated after measuring the weight of the 25 mm fluorocarbon-coated micro glass filter (FC-filter) before and after collection using a collector three times under an exposure of 4 hr to the test substance in the nasal exposure inhalation chamber. For particle size distribution, the mass median aerodynamic diameter (MMAD) for each exposure group was determined using a cascade impactor (Model-135 Mini MOUDI Impactor, MSP, MN, USA) during the exposure time. The delivered dose (DD) was calculated using the formula described by Alexander et al. [8]. C was the actual concentration. The respiratory minute volume (RMV) was set at 0.2 L/min, duration of exposure (D) at 240 min, and body weight at 0.3 kg and 0.2 kg for males and females, respectively. Because it was assumed that the MMAD was less than 2 μm and was 100% inhalable, the inhalable fraction (IF) was considered as 1.
Table 1 shows the actual concentration, MMAD, and DD results of the test substance during the exposure period. In the conditions of 5 mg/L, 1 mg/L, and 0.5 mg/L, the average concentrations were 3.29 ± 0.10 mg/L, 1.03 ± 0.03 mg/L, and 0.52 ± 0.03 mg/L, respectively. The MMAD values measured at concentrations of 1.686 μm, 1.540 μm, and 1.352 μm corresponding to particle sizes of 1–4 μm recommended by OECD Test Guideline 436. The DDs were 552.72, 173.04, and 87.36 mg/kg/day for males and 829.08, 259.56, and 131.04 mg/kg/day for females (Table 1). Since the inhalation route exposes all animals in the same group to the same conditions during the administration period, the actual dose of the test substance per unit body weight varies even if all animals inhale the same amount of the test substance. By calculating the DD, it was possible to present the actual dose inhaled by the test animal.
| Parameters | Conc (mg/L) | |||
|---|---|---|---|---|
| 5 | 1 | 0.5 | ||
| Actual concentration (mg/L) | 3.29 ± 0.10 | 1.03 ± 0.08 | 0.52 ± 0.03 | |
| MMAD (μm) | 1.686 | 1.540 | 1.352 | |
| DD (mg/kg/day) | Male | 552.72 | 173.04 | 87.36 |
| Female | 829.08 | 259.56 | 131.04 | |
At 5 mg/L, both sexes died on the day of exposure. Death was observed a day after exposure to 1 mg/L in three males and two days after exposure in one female. Death was observed 2 days after exposure to one male at a concentration of 0.5 mg/L (Table 2). Increased size and discoloration of the lung were observed in all animals that died during the observation period (Table 3). The surviving animal lost weight rapidly after exposure and never recovered until euthanized (Figs. 1 and 2).
| Conc (mg/L) | Find mortality (dead/total N) | Total mortality (%) | |
|---|---|---|---|
| Male | Female | ||
| 5 | 3/3 | 3/3 | 100.0 (6/6) |
| 1 | 3/3 | 1/3 | 66.6 (4/6) |
| 0.5 | 1/3 | 0/3 | 16.6 (1/6) |
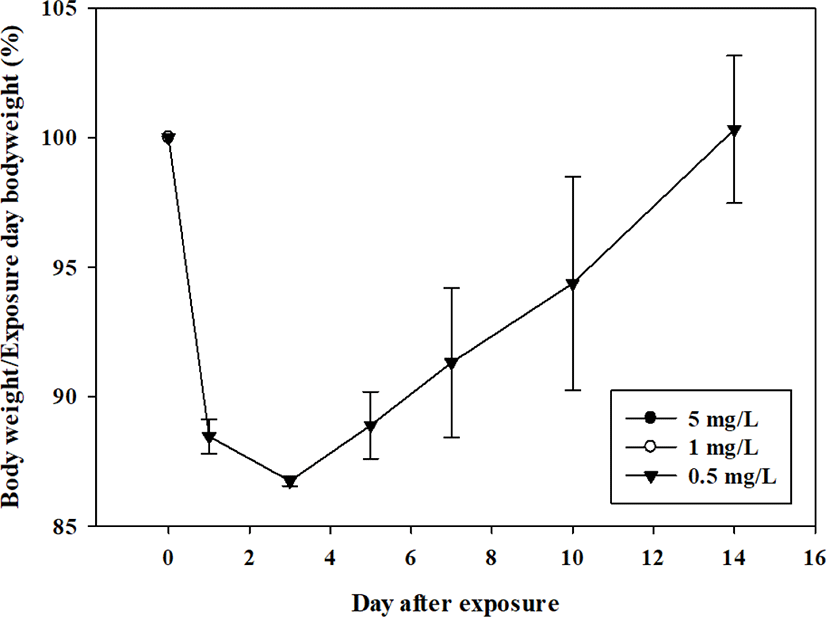
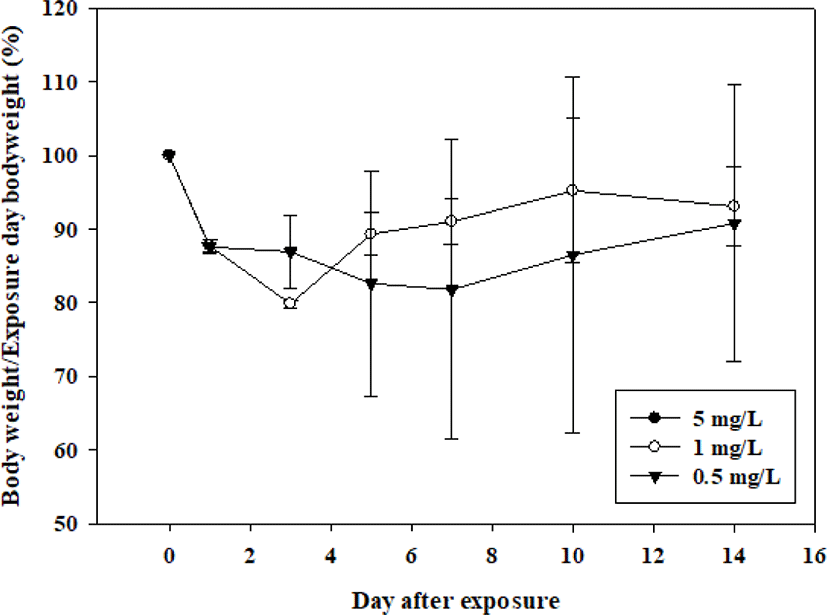
All observed changes were due to acute inhalation exposure to the test substance. Based on the above results, it was confirmed that 2-{2-[2-(4-nonylphenoxy)ethoxy]ethoxy}ethan-1-ol corresponds to the Globally Harmonized System of Classification and Labelling of Chemicals category 2 ( > 0.5–1 mg/L) when classified according to the OECD Test Guideline 436.
The mortality rate from acute inhalation exposure was higher among males than females. Sex differences in inhalation studies can be attributed to gender-related differences in body weight and increases in weight-related ventilation [7]. In contrast, a previous inhalation study that used a nose only inhalation exposure tube did not observe gender differences in the minute volume to body weights of 3-month-old rats [9]. As little is known about how sensitivity differs with sex and respiratory rate in test animals of the same age as the animals used in the study [8], the cause of the sex-related differences in inhalation cannot yet be determined. However, there is a concern that it may lead to under-classification due to sex differences depending on the test guideline used in the classification of acute toxicity of chemicals [10]. In consideration of this, an additional acute inhalation toxicity study using a fixed concentration procedure (OECD Test Guideline 433) using only one more sensitive sex is proposed.







