INTRODUCTION
An anaphylactic reaction is defined as a systemic, immediate hypersensitivity reaction most commonly caused by immunoglobulin E (IgE)-mediated immunologic release of mediators from mast cells and basophils [1]. This syndrome can affect virtually any organ in the body and can be categorized into cutaneous, respiratory, cardiovascular and gastrointestinal reactions [2]. The IgE-mediated reaction occurs within minutes to hours after antigen exposure [3]. Antigen-bound IgE interacts with Fc receptors on mast cells and basophils, causing these cells to degranulate, releasing molecules such as leukotrienes, histamine, eosinophilic chemotactic factor, platelet activating factor, kinins, serotonins, and proteolytic enzymes, which cause inflammation and thus tissue and cell damage [4, 5].
Vitamin K1 (VK1) has been widely used as a coumarin antagonist and for the treatment of hemorrhagic disease in practice [6]. Because of severe adverse drug reaction (ADR), intravenous injection of VK1 is not used. Therefore, VK1 is commonly administrated by the subcutaneous (SC) route in veterinary medicine. However, ADR to SC injection of VK1 has not been well characterized in comparison to that caused by intravenous injection of VK1 [6–8]. Indeed, to the authors’ knowledge, no reports have been published describing anaphylactic reactions after SC VK1 injection in dogs.
Thus, the aim of the present study was to determine whether anaphylactic reactions occur following SC VK1 injection. In this study, two cases of anaphylaxis after SC VK1 injection were identified, and then an experimental study was performed to further characterize these anaphylactic reactions.
MATERIALS AND METHODS
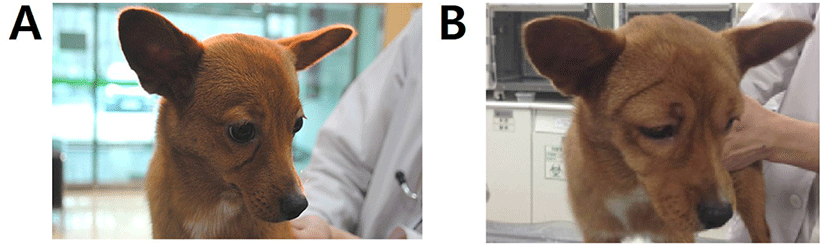
A 10-month-old, 3.54 kg, female mongrel dog (case 1, Fig. 1A) was presented with hematemesis and anorexia as principal complaints. A coagulation panel revealed a prolonged prothrombin time (48.3 sec; reference range 14–19 sec). After history taking and additional diagnostic tests, rodenticide toxicity was suspected, therefore the patient was treated with VK1 (2 mg/kg, SC, every 12 hr; Vitamin K1 inj., Dai Han Pharm, Seoul, Korea), esomeprazole (1 mg/kg, IV, every 12 hr) and maropitant citrate (1 mg/kg, SC, every 12 hr for 5 days) with hospitalization. At day 10, the patient showed severe facial edema (Fig. 1B) and the VK1 injections were stopped.
A 5-year-old, 3.14 kg, female Maltese dog (case 2) was referred because of abnormal liver enzyme activities and jaundice. A liver biopsy was planned to obtain a definitive diagnosis, but before this, serial SC VK1 injections were administered to prevent coagulopathy. The patient was given 2.5 mg/kg VK1, SC, every 8 hr for 3 days, and on day 7, a liver biopsy was performed. Four months later, the patient returned for an additional liver biopsy. However, a coagulation panel revealed a prolonged activated partial thromboplastin time (148.3 sec; reference range 75–105 sec) and prothrombin time (28.9 sec; reference range 14–19 sec), therefore VK1 (Vitamin K1 inj., Dai Han Pharm) was administered subcutaneously. Other medications such as ursodeoxycholic acid (10 mg/kg, PO, every 24 hr), silymarin (5 mg/kg, PO, every 12 hr) and D-penicillamine (10 mg/kg, PO, every 12 hr) were sustained. The following morning, the patient showed mild petechiation of the abdominal skin, but these lesions worsened, such that by 8 pm, generalized erythema and inguinal petechial signs were present. Anaphylaxis was suspected and therefore the VK1 injections were stopped.
Both of the dogs were treated with single intravenous injections of 0.5 mg/kg dexamethasone and 2 mg/kg chlorpheniramine, after which they recovered.
An experimental study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The procedures were approved by the Ethics Committee of Chungbuk National University (IAUCC, CBNUA-897-15-01). Six healthy, male beagles, aged one year, and with body masses of 9–12 kg, were included. The dogs were housed in individual cages with free access to water, and were fed a standard dry diet twice daily.
VK1 (Vitamin K1 inj., Dai Han Pharm) was subcutaneously injected into each dog (2 mg/kg) once daily for eight consecutive days. Blood samples were collected in heparin tubes on each day. Blood was centrifuged for 15 min after collection at 1,500×g at 4℃, and plasma was stored at −70℃ until assayed.
Any clinical signs that the dogs developed were recorded for 30 min after the administration of VK1 each day. Scores representative of the severity of each sign were recorded after drug administration, according to the assessment standard for anaphylaxis and anaphylactoid reactions (Table 1) [6]. The administration of VK1 was stopped when the scores increased.
The concentrations of plasma histamine and IgE were measured using commercially available canine-specific ELISA kits (Canine histamine [HIS] ELISA Kit, Cusabio Biotech, Wuhan, China; IgE Dog ELISA kit, Abcam, Cambridge, UK, respectively) according to the manufacturer’s protocols. All samples, standards, and controls were assayed in duplicate. The optical density was determined using an automated microplate reader (Elx808, Bio-Tek Instruments, Winooski, VT, USA) at 450 nm.
All statistical analyses were performed with a commercially available statistical program (Prism 6.01 for Windows, GraphPad Software, La Jolla, CA, USA). The Kolmogorov-Smirnov test was performed to evaluate the distribution of the data. All data are represented as median (range), and p<0.05 was considered statistically significant. The Wilcoxon signed rank sum test was used to compare plasma histamine and IgE concentrations of the six beagle dogs before and after VK1 injection.
RESULTS
Table 2 shows that almost all of the dogs did not display abnormal behavior or other clinical signs after the first VK1 injection. However, after repeated administrations, the dogs showed a number of clinical signs consistent with anaphylaxis, including skin scratching, sneezing, coughing, skin reddening, excess salivation, pawing the ground, rolling, and somnolence on days 4, 6, and 8 (Table 3). The days on which the most severe signs were observed varied between individuals.
Plasma histamine and IgE concentrations were analyzed before (pre) and after (post) SC administration of VK1 (data collected when the VK1 administration was stopped due to complications) to six dogs (Table 4). The median (range) of plasma histamine concentration significantly increased from 0.72 (0.49‒2.14) to 1.24 (0.51‒2.29; 95% confidence interval [CI] for the difference between medians = 0.02–0.40; p = 0.0005) after daily VK1 injection) µg/L. The median (range) of plasma IgE concentration also significantly increased from 3.31 (2.27–8.13) to 4.88 (2.35–12.4; 95% CI for the difference between medians = 0.01–2.99, p = 0.0490) µg/L concentrations were significantly increased after VK1 administration.
DISCUSSION
The lack of an acceptable standard definition and the wide variability in clinical signs complicates the diagnosis of anaphylaxis in veterinary medicine [5], meaning that is usually based on detailed history taking and clinical findings [5, 8]. Pertinent history includes recent vaccinations, transfusions, exposure to new foods, insect bite, and administration of medications [9]. Diagnosis depends on pattern recognition, specifically the sudden onset of characteristic signs after exposure to a known or potential stimulus, the time elapsed between exposure and the onset of signs, and the evolution of these signs over minutes to hours [5, 10]. Both cases described in this paper developed clinical signs after SC VK1 injection and recovered within a few hours of the last injection, following treatment with antihistamines and glucocorticoids. In each case, an anaphylactic reaction related to SC VK1 injection was diagnosed. Moreover, in the experimental study presented herein, mild clinical signs consistent with anaphylaxis and significantly increased concentrations of plasma histamine and IgE were detected after repeated SC injections of VK1. Therefore, we conclude that anaphylactic reactions can occur after SC VK1 injection, indicating that the SC route of VK1 administration might be not safe in dogs receiving the repeated administration of VK1.
In a previous study, histamine but not IgE concentrations significantly changed after intravenous VK1 administration, leading the authors to conclude that an anaphylactoid reaction had been induced [6]. Anaphylaxis is most often induced by repeated exposure to allergens, such as drugs, which can stimulate the body to produce antibodies through an IgE-mediated immune response. In contrast to anaphylaxis, anaphylactoid reactions are non-IgE-mediated and do not require a history of exposure [6]. However, both reactions are characterized by the release of the same molecules from basophils and mast cells, including histamine, beta-hexosaminidase, and tryptase [11, 12]. Therefore, anaphylaxis and anaphylactoid reactions are clinically indistinguishable.
It is unclear whether VK1 itself could cause an ADR. Because VK1 is a lipid-soluble substance, a solubilizing agent is used in the preparation for injection, typically polysorbate-80. The VK1 that was used in the clinical cases and in our experimental study also contained polysorbate-80 as the solubilizer. This solubilizer has been identified as the causative agent for the anaphylactoid reaction reported after intravenous injection [13, 14]. However, polysorbate-80 has also been shown to induce anaphylaxis in humans [7, 15].
Although an anaphylactoid reaction was observed after VK1 injection in a previous study [6], anaphylaxis occurred in the dogs described here. This disparity may be accounted for by differences in the route of administration: the previously reported anaphylactoid reaction may be specific to intravenous administration. Indeed, the route of administration of antigens is known to be important in determining the type of allergic response generated [16]. In addition, IgE-mediated and non-IgE-mediated hypersensitivity may develop according to the concentration of antigen present [13]. In the present study, 2 mg/kg of VK1 was administered, but a lower dose of VK1 (0.25 mg/kg) was administered in the previous study [6]. Thus, the development of contrasting reactions in the dogs in this study and those previously reported may be the result of differences in the administration route or dose of the VK1 preparation containing polysorbate-80.
There are some limitations to this study. Firstly, the number of dogs used in the experiment was very small. Secondly, we used an injectable formulation of VK1 containing polysorbate-80, which could itself induce ADRs, as described above. Ideally, administration of a VK1 preparation without a solubilizer would also be necessary to ascribe the effects to VK1, rather than to the solubilizer. Thirdly, we did not perform a basophilic activation test. This test measures the capacity of basophils to release histamine or upregulate activation markers, such as CD63 and CD203c, in response to an allergen [17, 18], but it is not readily available for veterinary use [19]. Lastly, we did not measure the plasma histamine and IgE concentrations in the two clinical cases. Although there are no universally accepted tests for diagnosing anaphylaxis, as described above, measurement of plasma histamine and IgE concentrations might be helpful in clinical cases.
In conclusion, the present study describes anaphylactic reactions resulting from SC VK1 administration in dogs. In some countries, an oral formulation of VK1 is not available; therefore, clinicians should be aware that repeated administration of VK1 with SC route could trigger an anaphylactic reaction in dogs.







