INTRODUCTION
Most patients with diabetes have a clinical symptom of diabetes-related hyposalivation [1]. The maintaining of normal function of the salivary glands is important for a healthy oral environment. The salivary gland hypofunction can induced several clinical discomforts, such as sallowing problem, xerostomia, and halitosis [2]. Moreover, this functional disorder of the salivary gland wersens the quality of life and increase the prevalance of various oral diseases, such as periodontitis, mucositis, and tooth decay [3]. The pathogenic risk factors of salivary gland hypofunction are chemical medications, several chronic diseases, radiation therapy, and aging process [4]. To control this clinical symptom, there are several treatment options, such as artificial saliva, chewing gum, malic acid and pilocarpine [5]. However, these medications only help temporary relif from symptom through an increased salivary flow rate.
Advanced glycation end products (AGEs) are non-enzymatic modifications of proteins or lipids with sugars. AGEs form in vivo in hyperglycemic environments and during aging [6, 7]. AGEs accumulate in various tissues and bind to AGE-specific receptors (RAGEs), which play an important role in the development of diabetes-related organ hypofunction [8]. Evidence shows that AGEs could cause the generation of reactive oxygen species (ROS) [9]. Cells in the human body are chronically exposed to oxidative stress during the development of diabetes, thereby causing cell injury [10]. The irreversible formation of AGEs increases the risk for the development of periodontitis, pulptitis in oral tissues [11]. RAGE is present in the salivary gland and highly expressed in the salivary gland with Sjögren’s syndrome [12]. However, it is not clear whether AGE accumulation is due to diabetes-related salivary gland hypofunction.
Polydatin (resveratrol-3-O-β-mono-D-glucoside) is a polyphenol that can be easily accessed from peanut, grape, and red wines [13]. Polydatin has potent anti-diabetic, anti-oxidant, anti-inflammation, anti-cancer, and cardiovascular protection effects [14]. However, its preventive role on diabetes-related salivary gland hypofunction has not been investigated. Thus, we examined the protective effect of polydatin on diabete-related hypofunction of the salivary glands in streptozotocin-induced diabetic rats.
MATERIALS AND METHODS
Diabetes was induced by the intraperitoneal injection of streptozotocin (STZ, 60 mg/kg, Sigma-Aldrich, St. Louis, MO, USA) in 7-week-old male Sprague Dawley rats (Koatech, Pyeongtaek, Korea). Normoglycemic control rats were administered vehicle only. Normoglycemic and hyperglycemic rats (blood glucose levels > 300 mg/dL blood) were randomized into the following 4 groups of 8 rats: normoglyemic control group (NOR), diabetic group (DM), polydatin (50 and 100 mg/kg/day)-treated two groups. Polydatin (Sigma-Aldrich) was given orally once a day for 4 weeks. To measure the salivary flow rate, rats were intraperitoneally injected with pilocarpine hydrochloride (2 mg/kg, Sigma-Aldrich). Saliva samples were then collected for 15 min. At necropsy, blood samples were collected, and submandibular salivary glands were isolated. All experimental procedures were performed under the supervision of our Institutional Animal Care and Use Committee (IACUC No. 2020-24).
The total protein levels in saliva and serum were examined using a Quick Start™ Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). Briefly, saliva samples were centrifuged at 13,000×g for 10 minutes at 4℃ to remove food residues. Total protein concentration in saliva and serum was determined by the Bradford method, using bovine serum albumin as a standard, and the plates were read at 600 nm in a microplate reader (Tecan, Männedorf, Swiss). The AGEs levels were detected using a rat AGEs ELISA kit (MyBioSource, San Diego, CA, USA) according to the manufacturer’s instructions.
A protein extraction solution (RIPA, Elpis Biotech, Daejeon, Korea) containing a HaltTM Protease Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA, USA) was applied to lyse the the submandibular salivary gland tissues. Afterward, a Protein Assay Dye Reagent Concentrate (Bio-Rad) was conducted to examine the extracted protein. The levels of ROS in the submandibular salivary gland tissues were examined using a Rat Reactive Oxygen Species ELISA Kit (MyBioSource) according to the manufacturer’s instructions.
Fixing process of submandibular salivary gland tissues was conducted to evaluate the tissue structure through the following steps: fixation in paraformaldehyde solution (4%), dehydration by using gradient ethanol, incorporation with paraffin, incision into smaller parts (4 µm), and staining with hematoxylin and eosin (H & E). The tissue sections were examined under a light microscopy (BX51, Olympus, Tokyo, Japan).
Apoptosis was determined using an in situ cell death detection kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. After paraffin removal, the cut sections were incubated in 100 μL volume of proteinase K (20 µg/mL) for 10 min at ambient temperature. Then, they were transferred into a TUNEL reaction mixture with a volume of 50 μL and placed in a dark incubator for 1 h at 37℃. A solution of Vectashield Mounting Medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Road Burlingame, CA, USA) was exerted to stain the prepared sections. The numbers of TUNEL-positive cells were counted under a fluorescence microscope (BX51, Olympus).
RESULTS
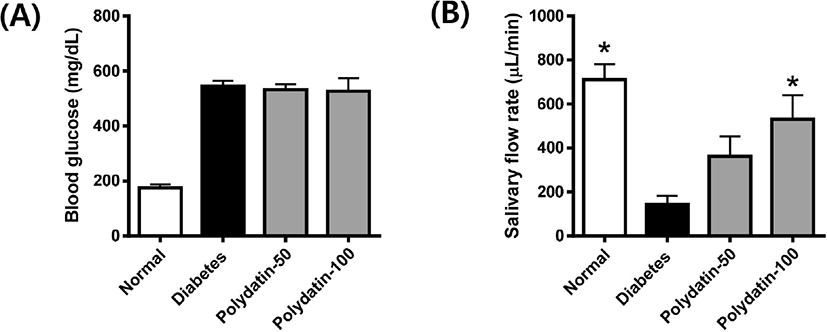
The blood glucose level in the STZ-induced diabetic rats was significanly increased compared with that in the control group and was not significantly altered by polydatin (Fig. 1A). The salivary flow rate was reduced in the STZ-induced diabetic rats, but reduced saliva secretion was significantly recovered following treatment with polydatin (Fig. 1B). These data suggest that diabetic rats had salivary hypofunction owing to decreased saliva secretion, which can be alleviated by polydatin.
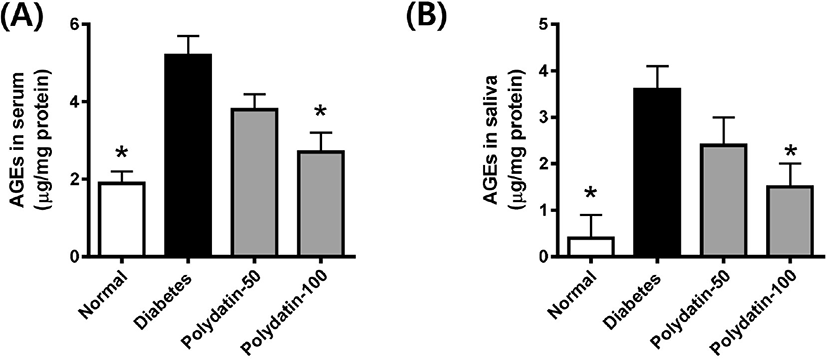
To determine whether polydatin reduced the AGEs formation in the body, we examined the levels of circulating AGEs in serum and secreted AGEs in saliva. As shown in Fig. 2, both circulating and secreted AGEs levels in the STZ-induced diabetic rats were significantly higher compared with the normoglycemic control rats. Polydatin decreased the levels of circulating and secreted AGEs compared with the DM.
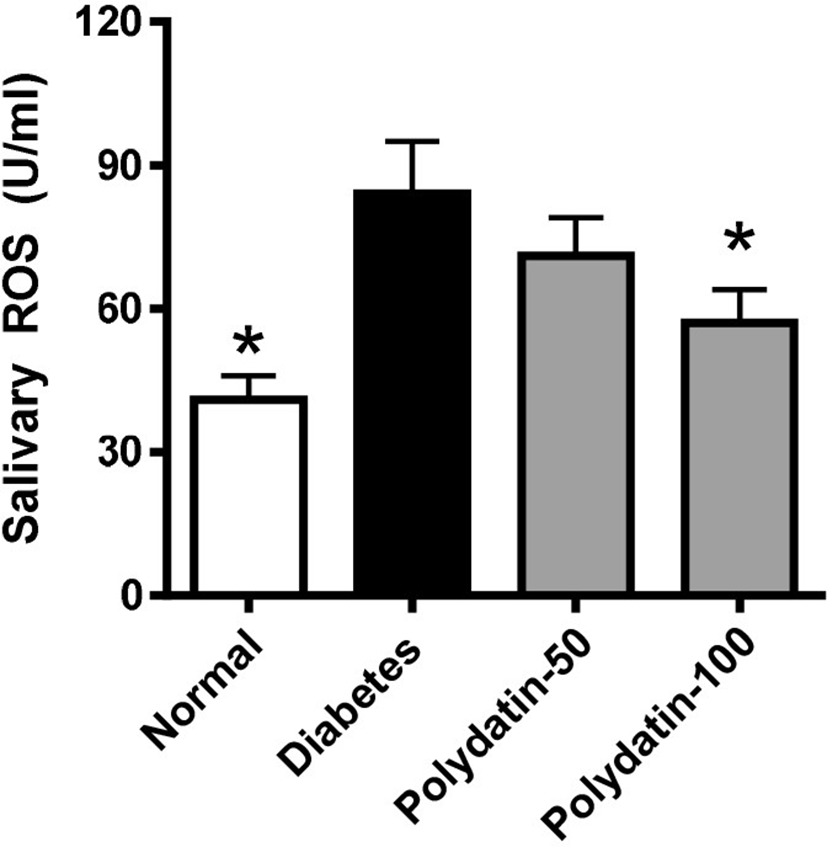
To evaluate the status of salivary oxidative stress in the STZ-induced diabetic rats, we examined the ELISA assay of ROS in the salivary gland tissues. As shown in Fig. 3, ROS generations were largely increased in the STZ-induced diabetic rats compared with the normoglycemic control rats. Polydatin significantly prevented ROS generation in the diabetic rats to a level similar to that observed in normoglycemic control rats.
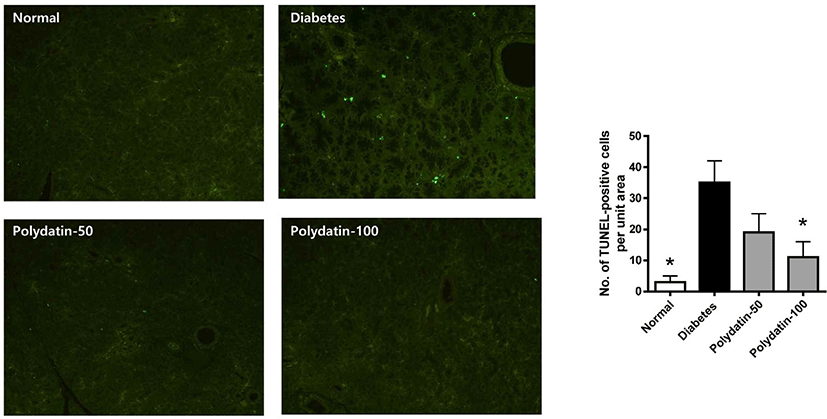
Our previous study reported that salivary acinal cells were histopathologically degenerated and damaged by apoptotic injury in the STZ-induced diabetic rats [15]. Thus, TUNEL staining was used to determine whether polydatin inhibits acinar cells die due to apoptosis. As shown in Fig. 4, apoptosis was detected by TUNEL staining and was increased in the DM and restored by polydatin. Polydatin protects salivary gland tissues by reducing apoptosis.
DISCUSSION
The gradual decline in salivary function is commonly observed in patients with diabetes. Patients with salivary hypofunction have a high risk factor for developing oral diseases such as tooth decay and periodontitis [16, 17]. Thus, the diabetes-related decline in salivary function should be managed to maintain a healthy oral environment. In this study, we assessed the effect of polydatin on hypofunctioning salivary glands by reducing AGEs burden.
Our study showed evidence indicating that enhanced AGEs burden occurred in the salivary gland of the STZ-induced diabtic rats. The functional alteration of the salivary gland was significantly induced in these diabetic rats compared with the normoglycemic control rats. These findings indicate that the significant AGEs burden in the salivary glands led to significant hypofunction of this tissue. This hyposalivation is in agreement with the results of Knaś et al. [18], who observed a decrease in the salivary flow rate and the enhanced oxidative stress in STZ-induced diabetic rats. Our results described the link between diabetes-related hypofunction of the salivary gland and AGEs burden. In addition, we showed that polydatin treatment exerts preventive effects on salivary glands in the diabetic rats.
The cytotoxic effects of AGEs have been shown in several studies [19]. AGEs can accumulate. Since the body does not contain any enzymes capable of AGE structural degradation, AGEs can accumulate in many tissues [20]. The interaction between AGEs and its receptor can induce the overproduction of ROS [21]. Oxidative stress is an important feature of diabetic complications [22]. ROS production was accelerated under diabetic conditions [10]. ROS frequently contribute to the tissue-damaging effects [9, 23]. Collectively, These results suggest that the diabetic conditions in the STZ-induce diabetic rats can induce salivary injury through AGEs-related oxidative stress and inflammation, which can lead to salivary gland dysfunction.
Here, we hypothesized that the reduction in AGEs burden by polydatin may contribute to the inhibition of salivary gland dysfunction. The current study clearly demonstrated that polydatin restored the levels of both circulating and secreted AGEs to near-normal levels in the the diabetic ats, in parallel with a marked increase of salivary flow rate. These findings provide evidence that polydatin has a beneficial effect on diabetes-related salivary gland hypofunction. Polydatin has the potent anti-glycation [24], and anti-oxidative activities [25, 26]. Chen et al. showed that polydatin inhibited the AGEs burden and renal injury in db/db diabetc mice [27]. In addition, polydatin enhanced tear production by attenuating oxidative stress in an animal model of dry eye disease [28].
Acinar cell loss by apoptosis can inevitably affect salivary flow rate, resulting in salivary hypofunction [29]. Moreover, high AGEs concentrations contribute to apoptotic cell death whenever they are generated in the context of the apoptotic process [30, 31]. The present study showed that diabetic conditions increased the number of TUNEL-positive cells in the salivary gland. However, we found that polydatin markedly decreased the number of TUNEL-positive cells in the STZ-induced diabetic rats. Similarly, polydatin has anti-apoptotic activity in neurodegenerative diseases [32], liver with acetaminophen-indced hepatotoxicity [33] and myocadial ischemia/reperfusion injury [34]. Thus, our findings suggest that polydatin has a potential anti-apoptotic effect in the salivary gland.
In conclusion, our study demonstrates that the AGEs burden was increased in the salivary gland of the STZ-induced diabetic rats. Polydatin has protective effects on the salivary gland of diabetic rats. These novel findings provide insight into the effects of polydatin against diabetes-related salivary hypofunction.







