Introduction
Hydropericardium-hepatitis syndrome (HHS) is characterized by severe hydropericardium, hepatitis and nephritis and has caused substantial economic losses in the poultry industry, especially in broiler chickens. After HHS was first reported in Pakistan in 1987, this syndrome has spread worldwide, including into Korea [1–3]. Among 12 serotypes, only fowl adenovirus serotype-4 (FAdV-4) can induce HHS, and this serotype has been identified in HHS outbreaks in Korea [1, 2, 4, 5]. Typically, 3–6-week-old chickens with HHS present with sudden death without any typical clinical signs, with mortality rates ranging from 30% to 70% [6, 7].
In previous studies, vaccines against HHS were developed, including live attenuated vaccines, inactivated vaccine and recombinant protein subunit vaccines [8–10]. In our laboratory, a FAdV-4 vaccine was developed based on a Korean FAdV-4 strain isolated from broiler breeder farms [11]. FAdV-4 vaccines offer broad cross-protection against other serotypes of FAdV that induce inclusion body hepatitis (IBH) in broiler chickens [9]. The need for FAdV-4 vaccine for prevention of HHS as well as IBH in the poultry industry has increased.
The pathogenicity of FAdV-4 in chickens differed based on various factors such as administration route, virus strain, host age, breed and immunosuppressive agents [12, 13]. In addition, inconsistent and milder clinical signs have been observed in animal experiments with FAdV-4 infection compared to those of HHS outbreaks in the field. Clinical signs or mortality were significantly variable in experimental specific-pathogen-free (SPF) chickens according to chicken age [2]. These results lead to difficulty in evaluating vaccine efficacy against FAdV-4 [11, 14].
Therefore, FAdV-4 SPF chicken models with significant clinical signs and mortality should be established for evaluating FAdV-4 vaccine efficacy. In this report, we comprehensively evaluate the pathogenicity of FAdV-4 in SPF chickens according to host age, virus passage number and virus titer. In addition, we apply the established SPF chicken model to evaluate newly developed live attenuated FAdV-4 vaccine candidates.
Materials & Methods
FAdV-4 ADL091024 was isolated from a layer flock with HHS in Korea. In brief, a 20% liver homogenate (LH) sample from laying chickens showing HHS was mixed with 1% penicillin G sodium (100 IU/mL) and streptomycin (100 μg/mL) (Sigma Chemicals, St. Louis, MO, USA) and centrifuged at 2,095×g for 10 min. The supernatant was collected after filtering using a 0.45-um pore size syringe filter (Millipore, Billerica, MA, USA) and frozen and thawed three times. Virus titration (50% tissue culture infectious dose, TCID50) was performed in chicken embryo liver cells (CELiC) derived from 14-day-old SPF chicken embryos (SPAFAS, Norwich, CT, USA) and determined using the Reed and Muench method [15]. Viral DNA was extracted from LH samples using the Viral Gene Spin Kit (Intron Biotechnology, Seoul, Korea). Polymerase chain reaction (PCR) targeting an 897-bp fragment containing the L1 loop of the hexon gene was performed using a primer pair from hexon A (5’-CAARTTCAGRCAGACGGT-3’) and hexon B (5’-TA GTGATGMCGSGACATCAT-3’) primers [16].
Ten 7-day-old embryonated SPF eggs were inoculated with 0.2 mL of ADL091024 strain via the yolk sac (YS) route. Eggs were candled twice daily for 10 days and embryos that died within 24 hours post-inoculation were discarded. At day 10 after inoculation, mortality and/or gross lesions such as stunting and curling, hemorrhage and liver lesions with mottling and necrosis were evaluated in embryos. Livers were collected aseptically, homogenized and centrifuged at 2,095×g for 10 min. The supernatant was obtained and then inoculated into SPF eggs. This procedure was repeated nine times.
A total of 160 SPF white leghorn chickens were hatched from SPF eggs (SPAFAS), divided equally into 8 groups (20 birds per group) and reared in HBC2 positive-pressure isolators (Threeshine, Daejeon, Korea). All animal experiment procedures were performed under the guidelines of Institutional Animal Care and Use Committee of the Animal and Plant Quarantine Agency. Four groups were inoculated intramuscularly at 1 day old, and the other four groups were inoculated at 14 days old. The inoculated samples were LH, viruses passaged four times (YS4) and nine times (YS9) in YS of chicken embryonated eggs, respectively (Table 1). Mortality, body weight gain, microscopic lesions and viral replication were examined in SPF chickens.
The selected challenge virus, YS4, was inoculated in a dose-dependent manner in 14-day-old SPF chickens (SPAFAS). Ten-fold serial dilutions of YS4 were prepared using sterile 1X phosphate-buffered saline. These virus preparations were designated as YS4 10−1 and 10−2 dilutions, respectively. Thirty-six 14-day-old chickens were randomly assigned to four groups of 9 birds. Birds in each group were intramuscularly inoculated with 0.2 mL of YS4, YS4 10−1 and YS4 10−2 dilutions. Another group of 9 birds was used as the negative control group, without virus inoculation. Mortality, body weight gain, microscopic lesions and viral kinetics were examined (Table 2).
An attenuated live FAdV-4 vaccine candidate was developed through serial passaging of the ADL091024 strain in CELiCs from 14-day-old SPF chicken embryos (SPAFAS). Vaccine efficacy was evaluated using the chicken FAdV-4 challenge model as follows. A total of 36 1-day-old SPF chickens were divided randomly into four groups (A, B, C, and D) of 9 birds. All birds were weighed before inoculation and two groups (A and B) were immunized with 4.54 × 106 TCID50/bird of FAdV-4 isolate ADL091024 per oral (PO) at the age of 1 day old. Two weeks after immunization, all chickens in group A and C were challenged with 6.31 × 108 TCID50 /bird of YS4 virus intramuscularly. All birds were maintained in HBC2 positive-pressure isolators and were clinically examined daily for 10 days after challenge (Table 3).
Clinical signs with mortality, mean body weight gain, gross lesions, histological lesions and viral DNA detection rate were identified for pathogenicity evaluation. All chickens were monitored daily for any clinical signs including depression, crouching position and mortality during 14 days post-inoculation (dpi). At 3, 5, 10, and/or 14 dpi, 10 chickens were randomly weighed and three and/or five chickens including dead birds from each group were euthanatized for necropsy. On necropsy, liver and heart were collected for histopathology. And liver and cecal tonsil (CT) were aseptically collected for high sensitivity of viral detection using PCR. The pathogenicity was assessed comprehensively based on clinical signs, mortality, gross lesions, histopathologic lesions and viral DNA detection rates. Microscopic lesions were scored in formalin-fixed liver and heart tissues. Score was determined based on lesion severity as follows: 0 = no lesions, 1 = focal, 2 = multifocal, 3 = diffuse histological lesions such as necrosis, lymphocyte infiltration and intranuclear inclusion body (IN/IB).
For statistical analysis, one way analysis of variance was used to compare the histopathological lesion scores using Microsoft Excel (Microsoft Office Excel 2010; Microsoft, Redmond, WA, USA). Statistical significance between groups was determined based on probability (p) values under 0.05.
Results
During serial passages of FAdV-4 (ADL091024) in the yolk sac of embryo, gross lesions including stunting, hemorrhage and white foci of the liver were observed in embryos at every passage. Finally, LH, YS4 and YS9 viruses were selected for further virus characterization and animal experiments. All challenge viruses from LH, YS4 and YS9 were positive on PCR examination and were classified into FAdV genotype C, serotype 4 in phylogenetic analysis (data not shown). This virus was most similar to the FAdV-4 reference L1 sequence of the KR5 strain [7]. The virus titers of the LH, YS4 and YS9 viruses were 6.31 × 107 TCID50/mL, 6.31 × 108 TCID50/mL and 4.73 × 108 TCID50/mL, respectively. The attenuated vaccine candidate titer was 4.54 × 106 TCID50/mL.
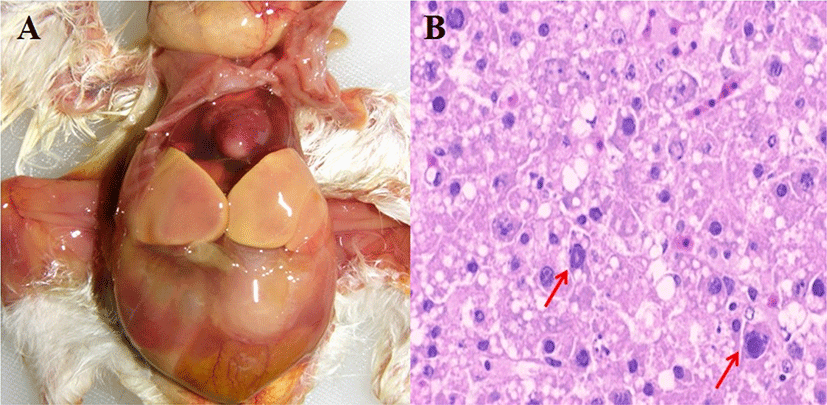
Three FAdV-4 viruses (LH, YS4 and YS9) with different passage numbers in embryonated eggs were inoculated into 1-day-old and 14-day-old SPF chickens via the intramuscular route. All challenge groups of 1-day-old chickens showed 100% mortality rates within 3 dpi and HHS pathognomonic gross lesions such as hydropericardium and hepatic necrosis (Fig. 1; Table 1). There were no data regarding mean body weight gain because all challenged 1-day-old chickens died within 3 dpi. In contrast, mortality rates were lower in 14-day-old chickens. Microscopic evaluation for the livers and hearts of 5 chickens from each group was conducted at each dpi. In 1-day-old chickens, histological lesion scores for liver and heart in challenge groups were significantly higher than the control group. The YS4 group showed the highest lesion scores, although this was not a statistically significant difference compared with other challenge groups. In 14-day-old chickens, histological lesions (such as IN/IB and lymphocyte infiltration) in the liver were more severe than those in the heart. Also, the YS4 and YS9 groups exhibited higher lesion scores than the LH group. FAdV-4 was detected in the liver and CT of 1-day-old and 14-days-old chickens on PCR, but not in the control groups. In the 1-day-old challenge groups, virus detection rates in liver were generally higher than those of CT. In particular, the YS9 group had the highest detection rates among the challenge groups. In 14-day-old challenge groups, there were detection rates more than 30% in the liver and CT of YS4 and YS9 groups.
Pathogenicity was comparatively evaluated among groups of 14-day-old SPF chickens with serially 10-fold-diluted viruses (Table 2). Only the group challenged with undiluted virus induced mortality of 11%, while there was no mortality in the other groups. Hepatic necrotic foci in livers was found in 56%, 33% and 33% of the 100-, 10−1-, and 10−2-diluted inoculation groups, respectively. Mean body weight gain was significantly lower in the no-dilution FAdV-4 inoculation group compared with the other groups. Significant histological lesions were observed in only livers, not hearts, in all inoculated groups. In PCR, FAdV-4 was identified in CT samples of all inoculation groups; however, in livers, molecular detection rates were 78%, 78%, and 67% in 100, 10−1, and 10−2 inoculation groups, respectively.
For evaluation of the efficacy and safety of the developed attenuated vaccine candidate, SPF chickens vaccinated at 1 day old and challenged with undiluted YS4 FAdV-4 at 14 days old were used (Table 3). Based on gross and microscopic lesions in livers and virus detection rates, there was significantly lower pathogenicity in group A than group C. Significant pathological characteristics were not observed in chickens inoculated with attenuated live vaccine without challenge (group B) or in the negative control group (group D) (Table 4). No mortality was observed in all groups.
Discussion
FAdV-4 infection occurs worldwide, including throughout Korea. Vaccination could be the best option for HHS prevention. However, no FAdV-4 challenge models have been established for evaluating newly developed vaccine candidates. In this report, we developed FAdV-4 challenge models for evaluating vaccine efficacy.
The severity of FAdV-4 infection is highly variable. Mortality rates range from 10% to 80% and 0% to 10% in broilers and layers, respectively [17, 18]. Consequently, we observed inconsistent results with regard to FAdV-4 pathogenicity in our chicken experiments. Some reports demonstrated that HHS could be reproduced by inoculation with FAdV-4 virus via an oral route in SPF chickens previously infected with immunosuppressive pathogens [1, 12]. Another report suggested that oral infection with some virulent FAdV-4 strains alone could induce HHS companied increased mortality in 2-week-old or older chickens [13]. This difference in severity among reports could be caused by several factors, including differences between the laboratory and field conditions, virus virulence, inoculation route and host age [14, 19, 20]. Korean FAdV-4 isolates could not reproduce clinical manifestations in 2- or 3-week-old SPF chickens [2, 11].
Inconsistent results make it difficult to evaluate precise vaccine efficacy and safety [11]. Therefore, we compared the pathogenicity of FAdV-4 isolates in SPF chickens according to virus passage number, host age and viral dose. For the challenge virus, ADL091024 strain was selected, which has a clinical history among HHS outbreaks in domestic laying chickens without co-infection of immunosuppressive agents such as chicken anemia virus (CAV) or infectious bursal disease virus (IBDV). In the field, high mortality are commonly seen in chickens aged <3 weeks old [21]. Therefore, ages of 1 and 14 days old were finally selected for this study. Our previous results revealed that there was no mortality or clinical signs in chickens challenged with LH samples without passage in vitro [11]. Therefore, in this study, FAdV-4 was passaged in chicken embryos via the yolk sac route nine times and pathogenicity was evaluated in comparison with FAdV-4 isolated with different passage numbers.
All groups of 1-day-old SPF chickens produced significant higher virulence, with similar severity to typical HHS clinical signs in the field regardless of virus passage number. However, significantly less virulence was observed in groups of 14-day-old chickens considering mortality, gross and microscopic lesions. These results indicate that host age has the important role in HHS manifestation in both experiments and real-world outbreaks. Among groups of 14-day-old chickens, YS groups showed higher mortality rates (20% in YS4 and 5% in YS9) than the LH group, which showed no mortality. Body weight gain, gross and histological lesions were also significantly more severe in YS4 and YS9 groups compared to the LH group. These results suggested that chicken embryo passage tend to increase the virulence of FAdV-4 in animal experiments.
Viral virulence has the tendency to be increased by viral loads in some viruses [14, 22]. Therefore, dose-dependent pathogenicity was analyzed in 14-day-old chickens with YS4 virus, which represented high virulence. Higher virus concentrations led to more severe virulence in chickens considering pathologic and clinical properties (Table 2). Comprehensively, 1-day-old chickens inoculated with YS4 at the highest virus titer presented with the most severe clinical signs in animal experiments.
Live-attenuated FAdV-4 vaccine was evaluated using the established challenge model. Outbreaks of FAdV-4 usually occur at 3–5 weeks of age. Therefore, immunization against FAdV-4 should be conducted in chickens <3 weeks old. No commercial live-attenuated FAdV-4 vaccine is available in Korea. Intramuscular injection with inactivated vaccine has limited practical use in 1-day-old chickens. Therefore, a new live-attenuated FAdV-4 vaccine was developed and evaluated in this report. The efficacy and safety of the newly developed vaccine candidate were successfully verified using an established chicken challenge model (Table 4). No pathologic characteristics were observed in vaccinated groups. Effective vaccine protection was identified based on comparable pathologic evaluation between vaccinated and non-vaccinated groups.
In conclusion, we established a FAdV-4 challenge model and successfully evaluated an attenuated FAdV-4 vaccine candidate using this model. This FAdV-4 challenge model will be useful for verification of newly developed FAdV-4 vaccine efficacy and safety.







