Introduction
Colorectal cancer (CRC) is a major cause of morbidity and mortality in the world. It accounts for over 9% of all cancer incidence and it is the third most common cancer worldwide and the fourth most common cause of death [1]. A lot of research about colon cancer and cancer-preventive materials has been done.
Colon cancer induced by Azoxymethane (AOM) + Dextran Sodium Sulfate (DSS) mouse model is similar to human colon cancer. The combination of DSS with AOM as a model for colitis associated cancer has gained popularity for its reproducibility, potency, low price, and ease of use. Studies in mice and rats have revealed that AOM/DSS-induced tumors display very similar features to human CRC even at the molecular level [2]. AOM has been shown to cause a K-ras gene transversion mutation from G:C to A:T at codon 12 deriving from O6-methyl-deoxyguanine adducts. K-ras is a small G-protein that regulates both MAPK and PI3K/Akt intracellular signal pathways, which, in turn, regulate cell growth, proliferation and glucose metabolism. AOM triggers break of cell cycle, thereby can cause carcinogenesis and AOM cause lipid peroxidation and generate malondialdehyde (MDA) in colon [3, 4]. MDA reacts with DNA and the DNA becomes mutagenic. It can cause carcinogenesis too [5].
AOM initiates colon cancer by inducing aberrant crypt foci (ACF). ACFs are the incipient lesions (which are defined as precancerous lesions of the colon [6]) which can observe and measure the development of colon cancer. ACFs can be observed by a microscope after stained with methylene blue [7]. When pre-neoplastic lesion is induced in colonic mucosa, ACFs looks like clusters of abnormal tube-like shape [8]. ACFs can advance to adenoma which can be invasive cancer sequentially [9]. DSS cause inflammation in colon and it can arouse colon carcinogenesis [10]. DSS used in this model can multiply pre-neoplastic lesions in colon as the following administration of AOM injection [11].
Stevia has been used throughout the world since ancient times for various purposes; for example, as a sweetener and a medicine [12, 13]. Stevia is a small perennial shrub that has been used for centuries as a bio-sweetener and for other medicinal uses such as to lower blood sugar. Its white crystalline compound (stevioside) is the natural herbal sweetener with no calories and is over 100–300 times sweeter than table sugar [12]. Stevia rebaudiana Bertoni plant extract, which contains anti-oxidant materials like anthocyanin and alpha tocopherol, is a beneficial and critical tool in sugar and calorie reduction, diabetes, weight management, and healthy lifestyles [12, 13]. In addition, the stevia plant extract has known to be antiproliferative to some cancer cell lines maybe due to its antioxidant activities [13–18]. In this study the effect of stevia was investigated on colon carcinogenesis induced by AOM/DSS in a mouse model and its antioxidant effect was also determined as MDA in feces of mice.
Materials and Methods
Stevia, which is extracted and purified from the Stevia rebaudiana Bertoni plant, was kindly obtained from Globalstevia (Cheongju, Korea). AOM was obtained from Sigma-Aldrich (St. Louis, MO, USA). DSS whose molecular weight is 36,000–50,000 was obtained from MP Biomedical (USA).
Male ICR mice (4 weeks old) were obtained from Samtako Bio Korea (Osan, Korea) and housed in an isolating polycarbonate cage (5 mice/cage). The temperature and relative humidity were set on 20±2°C and 50±20%. A light and dark cycle was set on 12 h each and the intensity of illumination was maintained at 150–300 lux. AIN-76A purified diet was obtained from Central Laboratory Animal (Seoul, Korea). During the experimental periods, diets and litter were all used after sterilization and the animal experiment was conducted in compliance with “Guide for care and use of laboratory animals” of Chungbuk National University (IACUC approval No.: CBNUA-1139-18-01). During the experimental period, body weight, food consumption and water intake were recorded weekly.
After male ICR mice (4 weeks old) were acclimated to cage for 1 week, 30 five-week old mice were divided into two groups including AOM/DSS group (control) and AOM/DSS + stevia-treated group (stevia group) (Fig. 1). AIN-76A purified diet was supplied to the mice. Stevia group was given distilled water containing stevia (0.5% of the original solution, ad libitum). Then, AOM (10 mg/kg b.w) was subcutaneously injected three times to mice at 0, 1st, and 2nd weeks of experimental period to induce the formation of pre-neoplastic lesion in colon (Fig. 1). At third week of the experimental period, distilled water containing 2% DSS was given for 7 days. Total experimental period is 6 weeks. At the termination of the study, mice were sacrificed by cervical dislocation to examine the colon.
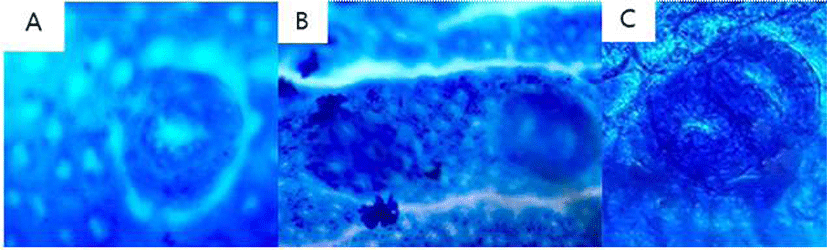
After colons were harvested, flushed with saline, opened longitudinally and fixed flat between filter papers in 10% neutral buffered formalin. The formalin-fixed tissues were stained in 0.2% methylene blue solution for a few seconds. The total number of ACF and the number of aberrant crypts (ACs) in each focus were counted under a microscope (40–100 ×). ACF were identified with following morphological characteristics: 1) the enlarged and elevated crypts than normal mucosa and 2) increased pericryptal space and irregular lumens (Fig. 2).
Dry feces were collected under each cage of 5 mice for 24 h before sacrifice. Sample was prepared by adding 1 mL of distilled water to 0.3 g of dried feces. Samples were incubated at 37°C for 1 hr and were thoroughly mixed during the incubation time. They were then centrifuged for 15 min at 20,000 g, and supernatant was collected and stored at –20°C until use. Malondialdehyde (MDA) levels were measured using TBARS Assay Kit (Cayman Chemical, 10009055). The method was performed on the basis of the reaction of the thiobarbituric acid (TBA) with MDA under high temperature (90°C– 100°C), then the resultant color was measured at 530 nm and MDA was calculated using a standard curve.
Results
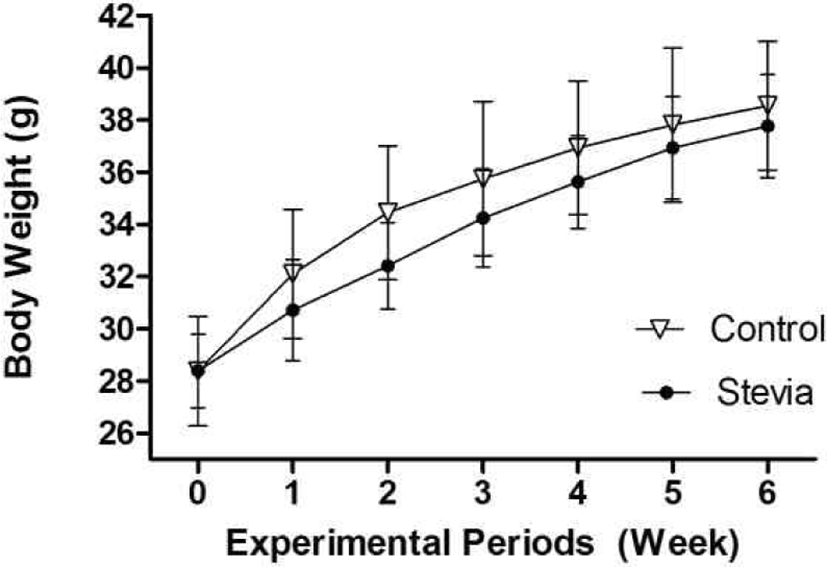
Body weight of the mice was increased as time goes by and there was a slight difference in body weights between control and stevia group (Fig. 3). After 1 week, the body weight of stevia group is consistently lighter than control group although there was no a significant difference between them.
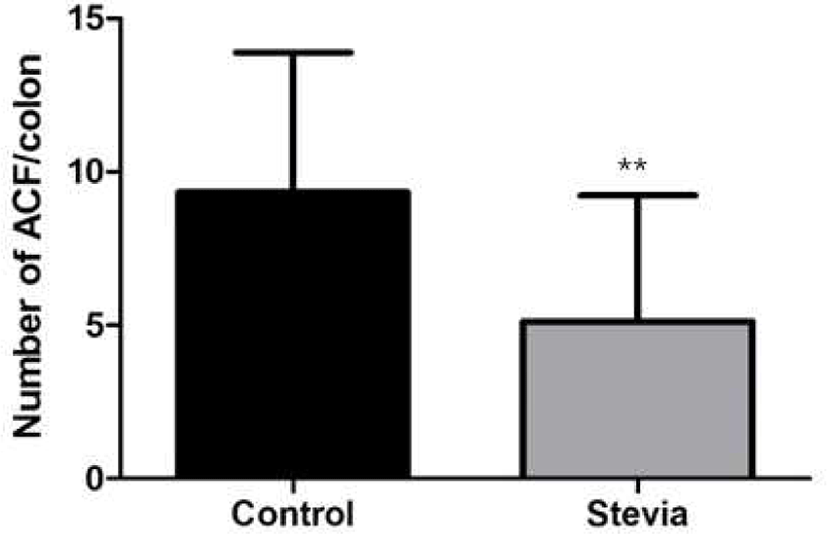
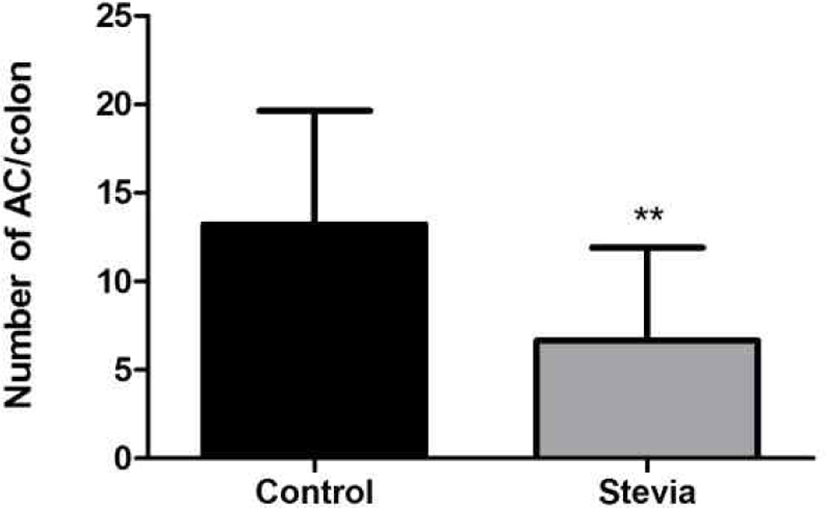
After the whole colons of mice were fixed with 10% neutral buffered formalin and stained 0.2% methylene blue solution, ACF and ACs were counted. The total number of ACF in stevia group (5.1 ACF/colon) was significantly low compared to control group (9.3 ACF/ colon) (p<0.01) (Fig. 4). Total number of ACs in stevia group (6.7) was also significantly low compared to control group (12.4) (p<0.01) (Fig. 5).
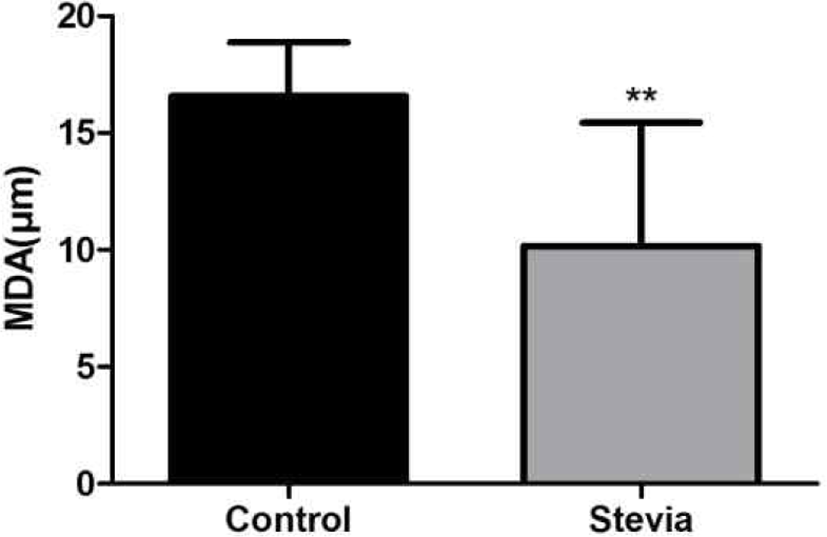
The 0.3 g of dry feces obtained from 5 mice for 24 h before sacrifice were prepared for TBARS assay of fecal water. The concentration of MDA, a product of lipid peroxidation, in stevia group (10.2) was significantly low compared to the control group (16.6) (p<0.01) (Fig. 6).
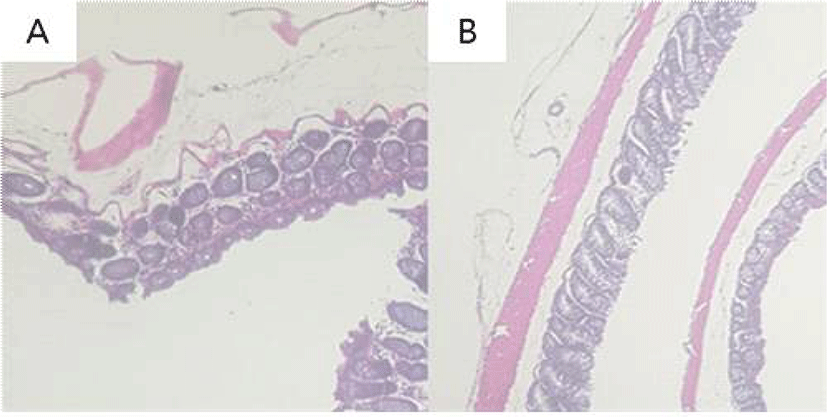
The colonic epithelia of mice were stained with H&E for histopathological examination. AOM/DSS-treated control group showed dysplasia, crypt damage, and inflammatory infiltrates in colonic mucosa. Stevia group showed less dysplastic change of colonic mucosa compared to the control group (Fig. 7).
Discussion
The materials which have preventive or deleterious effect about colon cancer is one of the important and rising issue. This experiment designed for finding whether stevia has a positive or negative effect on colon cancer prevention. The purpose of this experiment was to investigate that whether stevia decreases the possibility of colon carcinogenesis by counting the number of colonic ACF and measuring MDA with TBARS kit in colon carcinogenesis of an experimental mouse model. ACFs have some specific morphological features as a biomarker of colon carcinogenesis [8]. In the present study, the number of ACF in stevia group was significantly decreased by 45.2%, compared with control group. The number of ACs in stevia group was also decreased by 46%, compared with the control group. These results indicate that stevia protects against the carcinogenesis induced by AOM and DSS. The number of ACF in stevia group, which have more than 3 ACs (≥3 AC/ACF), was 2 whereas the number of ACF in the control group was 6. According to the study, the ACFs which have more than 3 ACs have great possibility to grow tumorogenic lesion [19]. Therefore, we can infer that possibility of great tumerogenesis is lower in stevia group than control group.
In vitro studies showed that glycosidic bond of steviosides cannot be hydrolyzed by human saliva, salivary α-amylase, pepsin, pancreatin, and pancreatic α-amylase, as well as jejunal brush border enzymes of mice, rats, and hamsters [13, 14]. However, the gut microbiota of humans, rodents, and hamsters are able to degrade stevioside to steviol. Steviol is not metabolized by gut microbiota and is absorbed from the intestine [13]. Stevia reduced IL-1β, TNFα and IL-1ra secretion prompted by HuCC colon cancer cells and enhanced IL-6 production induced by HuCC cells. The inhibition of pro-inflammatory- and increased anti-inflammatory cytokines release by stevia indicate that it possess valuable potential as carcinopreventive agents [15]. The stevia plant extract acts as an antioxidant being able to scavenge free radicals, but this activity was not due to stevioside [16]. The stevia plant extract clearly inhibited cyclin-dependent kinases (CDK4), which are key proteins in cell cycle regulation and proliferation, whereas stevioside did not, concluding that the antiproliferative activity of stevia may be due to inhibition of CDK4 performed by other compounds of the extract [16].
Although we did not measure antioxidant polyphenolic compounds, the proportion of polyphenols in the extract was 2.2% (mass concentration) calculated by the Folin– Ciocalteu method [17]. The ethanol extract of stevia plant was able to scavenge both DPPH and superoxide radicals in a dose dependent manner [17]. The extract exerts interesting antioxidant and antiproliferative effects compared to stevioside and that both activities might be mediated by other compounds like polyphenols alone or in synergy with steviol glycosides.
MDA is generated by lipid peroxidation which caused by AOM. In our study, lipid peroxidation in feces was evaluated by the TBARS assay. According to the study, MDA level is higher in AOM-injected mice group than normal group [4, 20]. The lipid peroxidation can be reduced with antioxidant such as vitamin E or anthocyanin [21]. The sites where antioxidant activity is low, have more possibility of generation of cancer than other normal site [22]. The MDA level from lipid peroxidation is a marker for oxidative stress [23]. According to the result of this study, level of MDA which suggest the level of lipid peroxidation is lower in stevia group than control group. This results suggest that the stevia extract used in this study has antioxidant activity and it has a protective effect against colon cancer induced by AOM in mice. In future, additional studies need to illustrate a detailed preventive mechanism of stevia on colon carcinogenesis.







