Introduction
Natural antibodies are produced against intrinsic proteins in vivo, rather than as a result of infection or vaccination with antigen from a specific pathogen. Natural antibodies produced from B1 cells are present in the abdominal and thoracic cavities and mainly include antibodies of the IgM isotype but also include IgG and IgA isotypes [1, 2]. In humans, anti-ABO blood group antibodies and anti-galactosyl antibodies are well-known natural antibodies [3, 4]. Recent studies have shown that these natural antibodies are actively involved in self-protection of living organisms, such as early suppression of pathogens, as well as removal of cellular debris and modified proteins [5, 6]. Furthermore, natural antibodies may be useful against cancer, atherosclerosis, and allergies [7–9] with effects of tumor surveillance and anti-inflammation. However, quantitative changes of natural antibodies and the mechanisms of their production are not well known.
The D-galactose (D-gal)-induced aging mouse model is prepared by subcutaneous administration of D-gal for approximately 6 weeks, once per day [10]. Excessive D-gal administration increases oxidative stress and causes accumulation of advanced glycation end products in vivo [11, 12]. This leads to various phenotypes of aging, such as cognitive dysfunction, nerve tissue damage, and liver disorders [13–15]. We previously reported that a phenotype of a reproductive disorder, polycystic ovary syndrome (PCOS), was induced in this mouse model [16]. In separate studies, we found that autoantibodies were produced against cross-reactive bovine serum albumin (BSA) in this model [17, 18]. Since then, we have focused on changes in autoantibody production and corresponding autoantigens in this mouse model.
In our preliminary experiments, serum from mice treated with D-gal (or not) was used for Western blotting analyses of whole tissue extract samples. Although some specific protein bands were observed when D-gal-treated serum was used, common protein bands at the same positions were detected in both sera, and these protein bands were detected more strongly in sera from D-gal-treated mice. In the present study, we investigated whether these common protein bands were due to natural antibodies. The antibodies present in serum samples from 3-week-old (3w) mice housed in specific-pathogen-free (SPF) conditions were defined as natural antibodies. We compared the amounts and characteristics of antibodies present in serum samples from 3w mice, 13-week-old mice (13w), and 13-week-old mice that were treated with D-gal for 6 weeks, beginning at the age of 8 weeks (13wDG).
Materials and Methods
Female ICR mice were used, Female ICR mice were purchased from Daehan Biolink (Eumseong, Korea). The mice were provided drinking water and a normal diet ad libitum and were maintained on a 12 hours light-dark cycle at 24 ± 1°C with 50% humidity. They were housed under SPF conditions. All animal studies were conducted in compliance with guidelines set forth by the Care and Use of Research Animals and were approved by the Animal Studies Committee of Dankook University (Approval number: DKU-17-002). The D-gal-induced aging mouse model was established as previously described [17]. In brief, 8-week-old mice were subcutaneously injected with D-gal (1,000 μg/kg mouse) once per day, and this was continued for a total of 6 weeks. As mentioned in the Introduction, these were considered 13wDG mice in this study. Thirteen-week-old mice (13w, as noted above) that were subcutaneously injected with Dulbecco’s phosphate-buffered saline (DPBS) alone were considered negative control mice. Three-week-old mice (3w, as mentioned above) were used as natural antibody control mice. Results are representative from three independent experiments with three to five mice per group.
Each serum sample from 3w, 13w, and 13wDG three mice was 3-fold serially diluted from 1:1500 to 1:121,500 with coating buffer (0.05 M carbonate-bicarbonate, pH 9.6) and 100 μL was added to wells of 96-well enzyme-linked immunosorbent assay (ELISA) plates. The serum was incubated in the ELISA plates, and then the plates were washed three times with washing buffer (0.05% Tween-20 in DPBS). The ELISA plates were incubated with blocking solution (3% BSA in DPBS) for 1 hr at room temperature. Horseradish peroxidase (HRP)-conjugated secondary antibodies against IgM, IgG, or IgA (1:2,000 dilution; Invitrogen, Carlsbad, CA, USA) were added to the plates and incubated at room temperature for 1 hr. The plates were washed, then TMB solution (Invitrogen) was added to each well and optical density values were measured at 450 nm using a Model 680 Microplate Reader (Bio-Rad, Hercules, CA, USA).
Calf thymus double-stranded DNA (dsDNA) antigen was diluted with DPBS, then added to each well in a 96-well plate at a concentration of 30 μg/mL and incubated overnight at 4°C Plates were washed three times with washing buffer and incubated with blocking buffer for 1 hr at room temperature. Each serum sample was diluted 1:200 and added to the plates, then incubated for 2 hours at room temperature. The remaining portion of the ELISA procedure was performed as described above.
Skin, spleen, and ovary tissues were harvested from an 8-week-old female mouse, then immediately frozen in liquid nitrogen and ground to a fine powder using a mortar and pestle. Tissue extraction buffer (Thermo Fisher Scientific, Inc.) was added to each sample (1 g/mL) and samples were incubated at 4°C for 30 min. The resulting lysates were centrifuged at 13,000 g at 4°C for 10 min and supernatants were collected.
In this study, tissue preparations were performed without perfusion. Therefore, to remove endogenous immunoglobulins that may be included in the tissue preparation, we carried out on 500 μL of sample with the addition of 50 μL of protein G sepharose and incubation for 1 hr with gentle agitation. After a brief centrifugation, the supernatant was collected. Protein concentrations of the tissue lysate supernatants were determined using the Bradford method. Then, proteins within these supernatants were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes.
Serums were obtained from 5 mice in each group and western blots were performed.
The membranes were incubated with blocking solution for 1 hr at room temperature. Serum samples from 3w, 13w, and 13wDG mice were diluted 1:1,000 in blocking solution and used as primary antibodies. The membranes were incubated with primary antibody solutions, overnight at 4°C. Then they were washed three times and incubated with secondary antibody (HRP-conjugated anti-mouse IgG, 1:10,000 dilution in blocking solution; Thermo Fisher Scientific, Waltham, MA, USA). Protein bands were detected using enhanced chemiluminescence reagents (Advansta, San Jose, CA, USA). When necessary, the X-ray film exposure time was extended up to 1 hr, considering the relatively low affinity of natural antibody.
Results
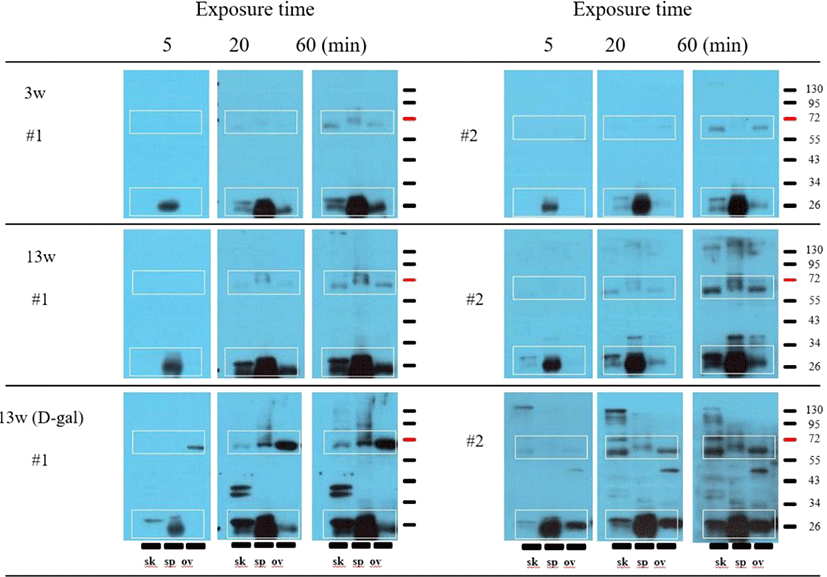
Western blotting analyses were performed with serum samples from two mice (#1 and #2) in each group (3w, 13w, and 13wDG). Only a few protein bands were detected at a relatively short exposure time of 5 min. However, additional protein bands were detected when the exposure time was extended to 20 min or 60 min. Bands were observed in nearly identical positions (white boxes) in each tissue at an exposure time of 60 min. These protein bands appeared to be sequentially amplified when using serum samples from 3w, 13w, and 13wDG mice. In particular, several specific protein bands, such as double bands around 40 kD, were detected in skin tissue using serum from 13wDG mouse #1 (Fig. 1).
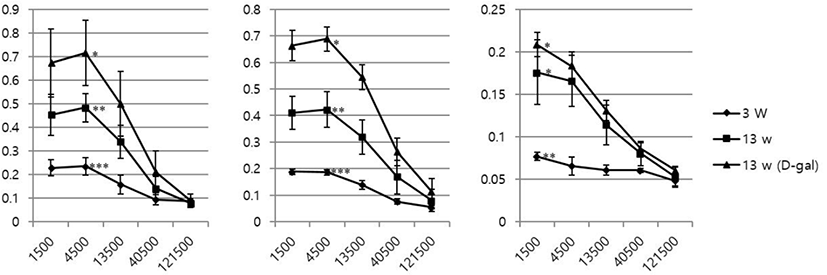
Total IgM, IgG, and IgA levels in serum samples of 3w, 13w, and 13wDG mice were examined by ELISA. The levels of all immunoglobulins increased in a sequential manner in 3w, 13w, and 13wDG mice (Fig. 2). This was presumably due to increased levels of natural antibodies, rather than increased levels of antibodies against unique infectious agents. There was a significant increase in all immunoglobulins among all groups, with the exception of IgA between 13w and 13wDG mice.
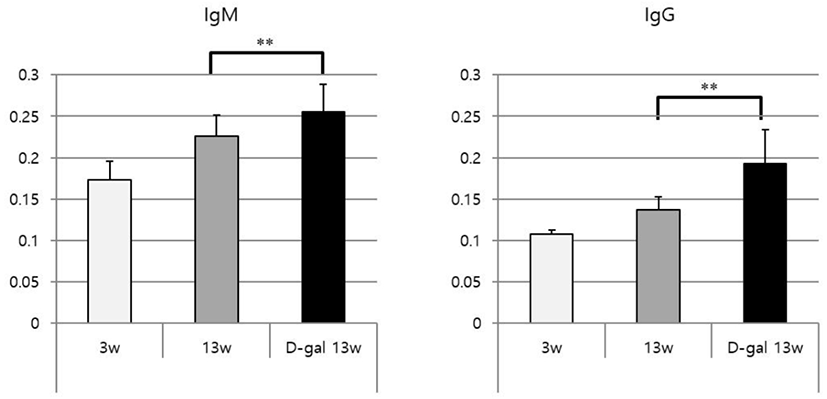
The levels of each antibody in each group of mice were measured using ELISA; the levels were significantly higher in 13wDG mice than in 13w mice (Fig. 3). However, the difference between 3w and 13w mice was not statistically significant.
Discussion
In previous studies, we observed that antibodies with cross-reactivity to BSA were produced in a D-gal-induced aging mouse model [17, 18]. We performed Western blotting of mouse skin, spleen, and ovary tissue lysates with serum samples from mice that were treated with D-gal, or not, to characterize in vivo antigens bound by these antibodies.
We selected these tissues in this study because skin is the site where it is repeatedly administered reducing sugar and it was the place where unknown antigen was confirmed by immunohistochemistry in the previous study [17]. And ovaries were selected because they were directly related to the phenotype of PCOS identified in previous studies using this animal model [19]. Finally, spleen was selected because it contains a large amount of blood and immune cells. We confirmed that serum antibodies in 13wDG and 13w mice bound to similar proteins on Western blots, although the degree of binding differed. In addition, these bands are not endogenous immunoglobulins (heavy or light chains) because they do not appear when western blot is performed by reacting only secondary antibody without first antibody (data not shown). Based on the results of this preliminary experiment, we performed the same experiment in the present study, adding serum samples from 3w mice to confirm whether the antibodies reacting with these common antigens were natural antibodies.
Similar protein bands were also detected in 3w mice, although the intensities of these bands were weaker (Fig. 1). These protein bands were amplified in sequential order as follows: 3w, 13w, and 13wDG mice. Furthermore, specific bands were identified, such as double bands with a molecular weight of approximately 40 kD in blots of skin tissue, when using serum samples from 13wDG mice. However, most protein bands were presumably based on binding of natural antibodies. The mechanism underlying such an increase in the concentration of natural antibodies in 13wDG mice cannot be explained by our findings. A recent study showed that the repertoire and quantity of natural antibodies increases by immunization [20]. In particular, 13wDG mice did not receive any immunity but produced a cross-reactive antibody to BSA. Therefore, it is possible that the mechanism related to the increase of the natural antibody by the immunity may have also worked in the 13wDG mice.
We have previously identified the presence of antibodies with cross reactivity to BSA in the same animal model. Increased natural antibodies that are further identified in this study are likely to differ from antibodies with cross reactivity to BSA. Antibodies with cross reactivity to BSA were removed in the course of treatment with a blocking solution containing BSA.
Changes in levels of total immunoglobulins (IgM, IgG, and IgA) increased in sequential order as follows: 3w, 13w, and 13wDG mice (Fig. 2). Because the mice were housed under SPF conditions, the increase from 3w to 13w may be a result of increased levels of natural antibodies, whereas the increase in 13wDG presumably includes both natural antibodies and anti-BSA cross-reactive antibodies. Generally, the sandwich ELISA method is used to measure amounts of immunoglobulins in serum. In the present study, a direct ELISA method was used in which proteins in serum were directly attached to an ELISA plate and detected using HRP-conjugated secondary antibodies against each immunoglobulin isotype. Quantitative differences in immunoglobulins among serum samples were observed at distinct dilutions (Fig. 2). Although it is difficult to know specific amounts, quantitative comparisons are possible. We presume that this method may help reduce time and cost, compared to that of the conventional sandwich ELISA method.
Antibodies against anti-dsDNA play an important role in autoimmune disease, and are considered representative anti-nuclear autoantibodies in patients with systemic lupus erythematosus (SLE) [21]. Currently, the dsDNA antibody test is frequently used for early diagnosis of SLE [19]. The presence of anti-dsDNA IgM antibodies has been confirmed in wild-type mice housed under SPF conditions, and these antibodies include polyreactive natural antibodies [22]. Therefore, we examined the levels of antibodies against dsDNA. IgM and IgG increased in a sequential manner in 3w, 13w, and 13wDG mice, and levels of anti-dsDNA antibodies also increased with age. Taken together, our results indicate that levels of natural antibodies increase in mice subcutaneously injected repeatedly with D-gal (D-gal induced aging mouse model). Berghof et al. reported that an increase in natural antibodies with age in a study using poultry [23].







