Introduction
Digestive diseases are a major issue in the swine industry worldwide. Diarrhea in piglets results from complex interactions among viral and bacterial pathogens, and environmental factors. The porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), and rotaviruses are common viral pathogens that cause piglet diarrhea. Other diarrhea-inducing pathogens include the bacteria Escherichia coli and Clostridium spp., and parasites such as coccidia [1]. Recent studies have also revealed other pathogens that can cause diarrhea in piglets [2,3]. Some microbes, such as Enterococcus spp. can proliferate in the gut and cause digestive problems [4].
Enterococcus spp. are Gram-positive, anaerobic bacteria that are present in the environment, as well as in the gastrointestinal tract of several animal species including pigs [5,8]. To date, 19 Enterococcus species have been identified, including Enterococcus (E.) faecalis and E. faecium, which cause most of the Enterococcus infections [9]. E. faecium species group typically adheres to the apical surface of enterocytes [8]. Thus, this species group including Enterococcus durans and Enterococcus hirae may cause diarrhea in suckling mammals [10–13]. Additionally, E. durans has been implicated in a few cases of piglet diarrhea in Korea [10] and Canada [14]. However, infection by E. hirae is rare [15]. In this study, we report on an outbreak of diarrhea associated with E. hirae in piglets from a farm in Korea.
Case Presentation
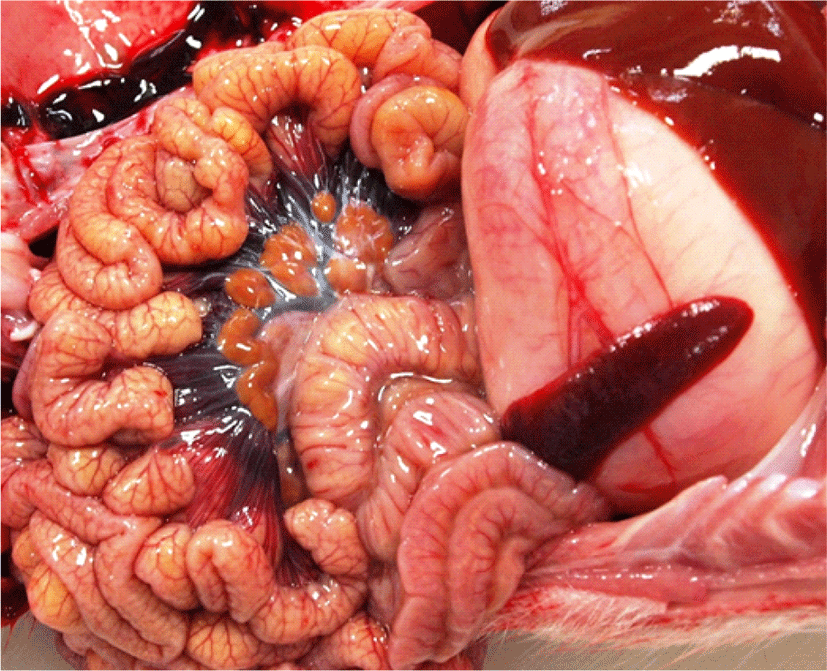
In 2017, four 4–5-day-old piglets with diarrhea from a farm in Korea were sent to the diagnostic laboratory at Optipharm Inc. (Cheongju, Korea). Although the piglets were continuously treated with antibiotics at the farm, their diarrhea did not improve. The piglets were thin and had rough and greasy coats. No disease lesions were observed during the initial post-mortem examination. The stomachs were filled with food material, and the wall thickness of the small intestines appeared normal. Furthermore, the omentum of the intestinal mesentery in each piglet was clearly observed, indicating that the piglets did not experience difficulties with milk intake (Fig. 1). The contents of the small and large intestines were fluid, and kidney stones were scattered across the bilateral sections of the kidneys (unpublished data), indicating that the piglets were severely dehydrated.
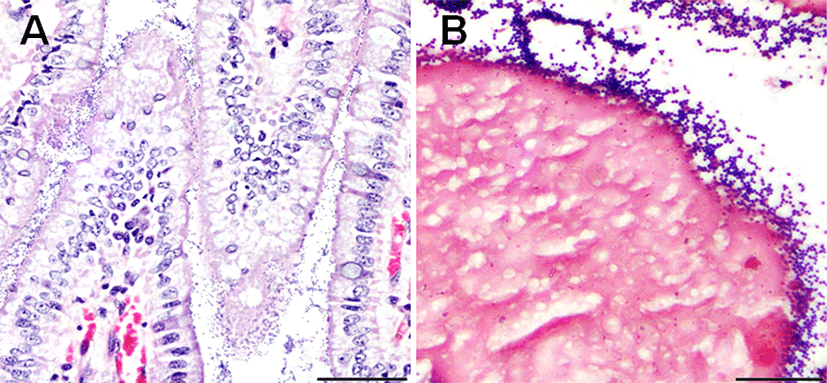
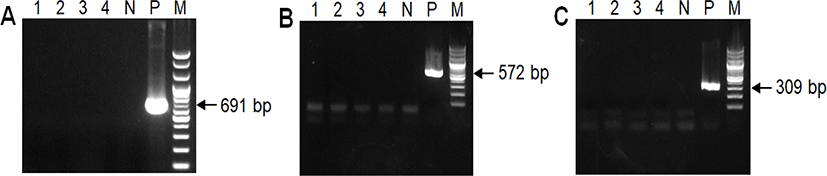
Tissue samples were collected from the jejunum, ileum, and colon of each piglet and fixed in 10% neutral buffered formalin for histopathological examination. The fixed tissues were processed, embedded in paraffin wax, and cut into 3-μm-thick sections. Tissue sections were stained using hematoxylin and eosin and Gram staining. Upon examination, numerous Gram-positive cocci were found to be adhered to the villous epithelial cells throughout the jejunum and ileum of each piglet. However, we did not observe significant changes in villi structure, such as in villi length, in the small intestine, nor any physical damage to the intestinal epithelium (Fig. 2). Polymerase chain reaction (PCR) was performed on small intestine samples to check if diarrhea-causing viral pathogens, such as PEDV, TGEV, and other rotaviruses, were present. Primer sequences and sizes are listed in Table 1. According to the PCR results, these viruses were not present in the piglet small intestines (Fig. 3). PCR results and histopathological examination also revealed that protozoan parasites, such as Isospora suis, were absent from the intestinal contents and intestinal villi (unpublished data).
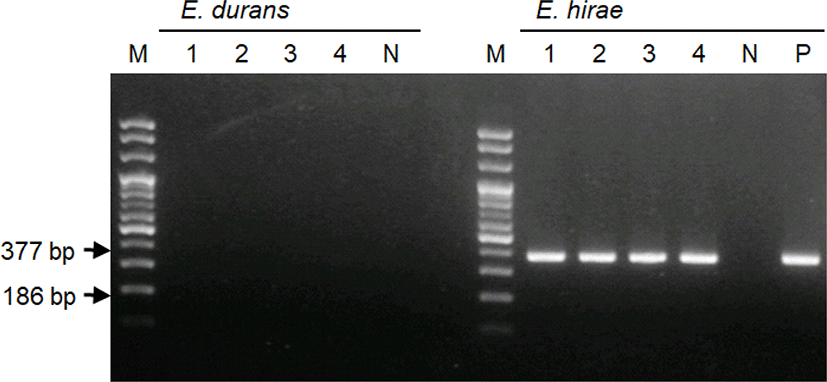
Samples of the intestinal contents of all piglets were inoculated on a sheep blood agar plate and incubated aerobically at 37°C for 24 h. The bacterial colonies produced were small and round, and induced α-hemolysis. The VITEK 2 system (bioMérieux, Marcy-l'Étoile, France) was used to identify the bacteria according to their biochemical properties. The results revealed that 99% of the bacterial colonies were E. hirae. PCR was also performed using DNase/RNase-free distilled water according to the method described by Knijff et al. [16], to identify the cultured colonies more accurately. PCR primers and reaction conditions are summarized in Table 1. Gel electrophoresis was performed using the PCR products, and bands corresponding to E. hirae were present in all four samples, whereas those of E. durans were not present (Fig. 4).
Lastly, we performed an antibiotic susceptibility test using the disk diffusion method. E. hirae was inoculated onto blood agar plates, and discs containing antibiotics (18 types) were placed onto the plates. Plates were then incubated at 37°C for 24 h. E. hirae was susceptible only to amoxicillin and florfenicol, and was resistant to several antibiotics that are used to treat enteric diseases, such as colistin, enrofloxacin, gentamicin, linsmycin, neomycin, tiamulin, trimethoprim/sulfamethoxazole, and tylosin (Table 2).
Discussion
In general, when diarrhea in piglets is caused by viral enteritis, the villi become shorter and physical damage to the epithelium in the small intestine can be observed by histopathological examination. We did not observe structural changes in the villi or epithelium of the small intestines of any of the piglets. Thus, the diarrhea was not caused by viral infections. Additionally, Gram staining did not reveal the presence of Gram-negative bacteria, such as Escherichia coli, which can cause enteritis without damaging intestinal villi. By contrast, numerous Gram-positive cocci were attached to the intestinal villi. These cocci also exhibited the same morphology as E. hirae. However, E. hirae is difficult to differentiate from E. durans using conventional methods, and their 16S rRNA is 98.8% similar [17]. Specialized diagnostic methods are needed to detect overexpression of specific microorganisms in the small intestine, in the absence of a gold-standard method [18]. In this study, we combined histopathological and microbiological methods to accurately diagnose an E. hirae infection. Thus, we used the VITEK 2 system and PCR to identify E. hirae, and diagnosed the piglets with diarrhea caused by E. hirae infection.
The main determinants of piglet diarrhea include passive immunity to colostrum and milk [19], environmental temperature [20] and humidity [21], management practices, and infections by specific pathogens [22]. Case histories should always be examined, as piglet diarrhea can be caused by a multitude of interacting factors [23]. In pig farms, antibiotics are commonly used to treat piglet diarrhea. The diarrhea in the four piglets in this study did not improve despite continuous antibiotic treatment; this could have been because E. hirae is resistant to most types of antibiotics. Although antibiotics help to prevent and treat clinical diseases, their continuous use in the swine industry has led to reduced microbiota diversity in pigs and the emergence of antibiotic-resistant bacteria [24]. Furthermore, exposure to antibiotics immediately after birth may affect the richness and diversity of intestinal microorganisms. As antibiotic-resistance genes accumulate in the bacterial community, the populations of certain species may start increasing [25]. Bacterial overexpression in the small intestine can inhibit enzymatic action, absorption, and normal metabolism, resulting in digestive or absorptive disorders [26,27]. Therefore, the continuous use of antibiotics may increase the risk of diarrhea in piglets caused by E. hirae, although this species is rarely pathogenic.
In conclusion, the pathoanatomical and histopathological characteristics of individual cases, and bacterial biochemistry, should be evaluated together with case history (e.g., antibiotic treatment) to determine the cause of diarrhea in piglets. Thus, this study presents rare cases of E. hirae infection of the piglet small intestine, which can occur in association with diarrhea possibly by the continuous use of antibiotics. However, further studies are needed to elucidate how E. hirae infection causes diarrhea, and to determine the relationship between antibiotic use and E. hirae overexpression in the small intestine.







