Introduction
As many chemicals have been developed, it is necessary to develop rapid in vivo screening methods with high-throughput analyses. Conventional rodent models in toxicity studies have some limitations for high-throughput detection of toxicants. In particular, carcinogenicity assays using rodent models take about two years, so it is necessary to develop a screening model that can test potential carcinogens in a shorter period of time.
Among many alternative animal models, zebrafish has traditionally been used in phenotypic-based screens and developmental biology. Recently, zebrafish has been used for rapid screening of many chemicals in vivo [1]. There is trend to expand the use of the zebrafish from acute to chronic toxicity studies [2]. The characteristics of zebrafish seem to make them an invaluable platform for screening chemicals before exposure in mammalian systems [3]. They are small, inexpensive to maintain and easy to breed to large numbers, resulting in low experimental price and compliance with 3R principles. Their physiology and development parallels that of mammals. They can live for several days in a single well of standard 6 or 12-well plates, surviving on nutrients stored in their yolk sac and absorbing compounds in the surrounding water through their skin and gills.
Unique biological insights from zebrafish have already led to the investigation of carcinogenesis markers used to treat cancer in humans. It has been reported that young zebrafish was most sensitive to 7,12-dimethylbenz[a]anthracene [4], and a variety of mesenchymal neoplasms occurred in zebrafish following exposure to N-methyl-N'- nitro-N-nitrosoguanidine, including chondroma, hemangioma, hemangiosarcoma, leiomyosarcoma, and rhabdomyosarcoma [5]. For studying mechanistic aspects of the carcinogenesis process, zebrafish appear to be a valuable model system that can greatly contribute to understanding of cancer biology and treatment [6]. Furthermore, comparative analysis of microarray data from zebrafish liver tumors with those from human tumor types revealed similarities at the molecular level [7].
Diethylnitrosamine (DEN) as well-known as hepatocarcinogen, is widely used in several strains of rodents [8–11]. However, though the zebrafish has been long advocated as a model for cancer research, little is known about cell death in zebrafish after DEN treatment. The transparency of early stage zebrafish larvae allows them to be viewed and monitored with fluorescent microscopy. Generally, the high tissue penetration capabilities of fluorescence bioimaging has enabled detection of biological processes during disease progression [12].
Taking into account the above-mentioned findings, this study was undertaken to investigate cell death induced by DEN treatment in zebrafish larvae in vivo using fluorescence bioimaging. Histopathological examination was carried out to confirm cell death.
Materials and Methods
Adult male and female zebrafish (Danio rerio) were obtained from Woojung Co. Ltd. (Suwon, Korea) and maintained at 25℃ to 28℃. For the week preceding the start of the study, they were acclimated to 12 h light and dark periods. They were fed TetraMin Tropical Flakes (Tetra USA, Blacksburg, VA) twice a day. Mortality rate was checked daily.
Fertilized eggs obtained from mating adult zebrafish were maintained in a freshwater tank at 28℃, and the developing ones were collected and maintained at 28℃. After seventy-two hours fertilization, the zebrafish were divided into five groups: group 1 (G1) as control, group 2 (G2) as probe control, groups 3, 4, and 5 (G3, G4, and G5) as DEN-treated at doses of 25, 50, and 100 μg/mL, respectively. At twenty-two hours after DEN treatment, groups 2, 3, 4, and 5 were treated with ApoFlamma H 675 (BioActs) at a dose of 100 μM/zebrafish. G1 zebrafish larvae were treated with equal volumes of PBS at the same time. This study (NSU-18-04) was approved by the animal experiment committee of Namseoul University based on the Animal Protection Act.
Before fluorescence in vivo bioimaging, the zebrafish were anesthetized with MS-222 (Sigma-Aldrich) at twenty-four hours after DEN treatment. Fluorescence bioimaging was conducted via fluorescence stereomicroscope (Neoscience, Suwon, Korea). Fluorescence intensity in region of interest (ROI) were recorded and the images were analyzed.
After fixation in 10% cold formalin, the zebrafish was transferred to ethanol for 24 hours and processed with slight modification as previously reported [13]. They were then embedded in paraffin. Sections (5 mm) were deparaffinized and the slides were dehydrated, with a wash for each step: xylene, 100% ethanol, 95% ethanol, 70% ethanol and distilled water. They were stained with hematoxylin and eosin (HE) for histopathological examination. The sections were then washed slowly under running distilled water for 10 min, dehydrated, mounted, and examined by light microscopy.
TUNEL assay was used to check cell death in the livers of the zebrafish. Tissue sections were dewaxed with xylene, hydrated and treated with proteinase K and 3% hydrogen peroxide. They were treated with TdT enzyme and anti-digoxigenin peroxidase conjugate in In Situ Apoptosis Detection Kit according to manufacturer’s instructions (Chemicon International, Temecula, CA). Avidin-biotin complexes were visualized using DAB substrate (Sigma).
Results
After anesthesia, zebrafish was placed horizontally and imaged by fluorescence stereomicroscope. Fluorescence intensity was detected in the livers of DEN-treated zebrafish (Fig. 1).
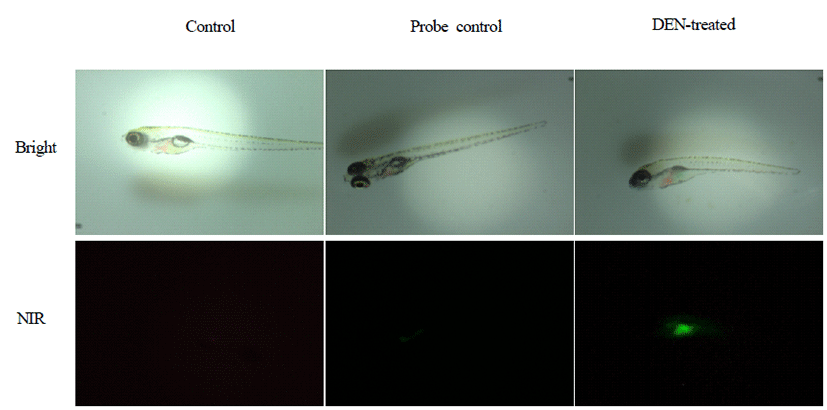
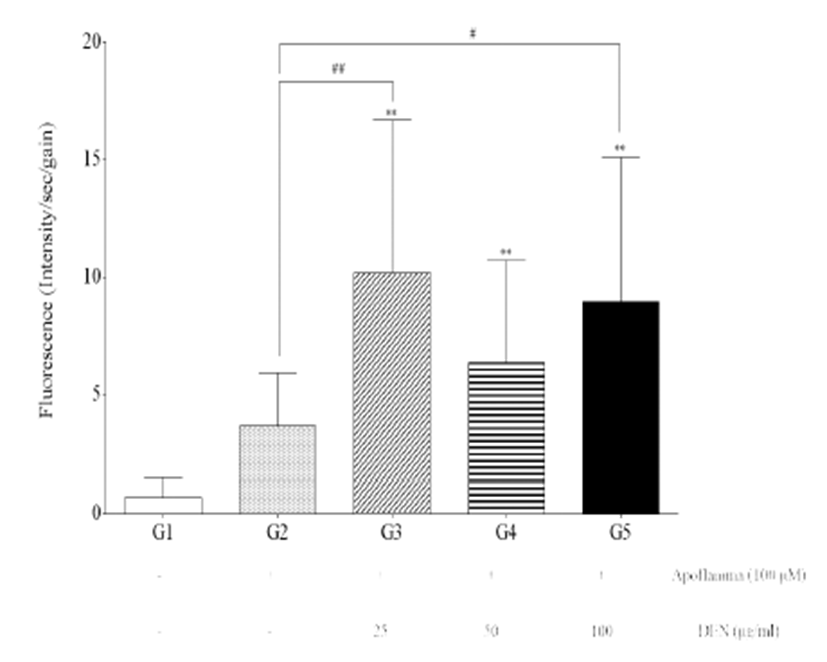
Fluorescence intensity of the livers of G3, G4, and G5 was significantly increased compared with those of G1 (p<0.01) (Fig. 2). Furthermore, fluorescence intensity of the livers of G3 and G5 was significantly increased compared with those of G2 (p<0.01 and p<0.05).
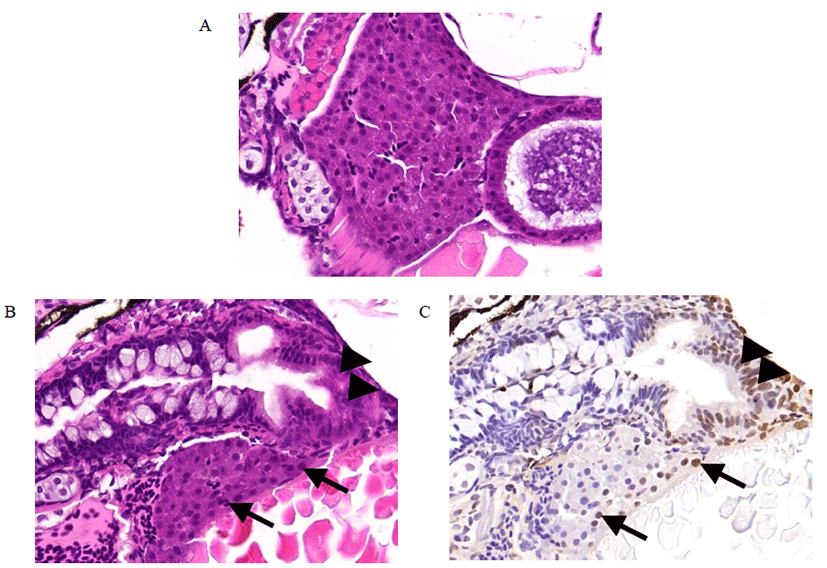
Liver tissues from DEN-treated zebrafish were assessed histopathologically by HE staining and TUNEL staining. HE staining showed that normal liver architectures were seen in the control (Fig. 3A). However, cell deaths were observed in DEN-treated zebrafish (Fig. 3B). TUNEL staining confirmed cell death in the same location (Fig. 3C).
Discussion
In this study, treatment with DEN from 96–120 hours post fertilisation induced hepatic cell death in zebrafish. It seems that young zebrafish may be sensitive to DEN treatment. It was reported that DEN treatment induced a higher incidence of liver tumors in young rodents than older ones [14], which was associated with the proliferation rate of liver cells in young animals. However, there have been no reports about DEN-induced cell death in young and adult zebrafish. In mice, the death of DEN-exposed hepatocytes appears to activate adjacent myeloid cells to produce mitogens that promote compensatory proliferation of surviving hepatocytes [15]. Moreover, liver injury in response to DEN exposure elicits an inflammatory response in nonparenchymal cells, which secrete several cytokines and growth factors that promote compensatory proliferation of quiescent hepatocytes carrying DEN-induced mutations [16]. It was reported that stem cells are located in the canals of Hering in the liver [17], which might be related to carcinogenic initiation from liver stem cells [18] during carcinogen exposure. It was also reported that that cell death might promote early tumorigenesis [19, 20]. However, there have been no reports about the role of stem cells in hepatocarcinogenesis of zebrafish. Further studies are warranted to investigate cell death after carcinogen treatment and the role of stem cells according to the developmental stage of zebrafish. In this study, fluorescence in vivo bioimaging revealed a significant increase in fluorescence intensity in zebrafish treated with DEN compared to control zebrafish, showing quantification of cell death in the animals’ livers. This provides evidence that early exposure of DEN induces cell death. For detection of cell death, fluorescence in vivo bioimaging was used to visualize the target molecule. It has been reported that there is a limitation to detection of fluorescence signal in intact rodents, because of the depth limitation of fluorescence bioimaging. As zebrafish is transparent, it is easier to detect fluorescence signal throughout the body. Among many methods for the detection of cell death, histopathological examination is a gold standard tool. HE staining has several unique advantages over immunofluorescence. However, it has some limitation steps, largely owing to specimen manufacturing, time of fixation, processing, embedding and staining. To overcome these time consuming steps, fluorescence in vivo bioimaging was applied to detect the pathological alterations in live zebrafish in real time. Further studies are warranted to investigate cell death after carcinogen introduction using transgenic zebrafish.
Many fluorescence bioimaging agents have been developed to detect several physiopathological alterations [21]. In this study, Annexin-Vivo 750 treatment makes it possible to view cell death in zebrafish. Annexin-Vivo 750 is a fluorescence imaging agent for the detection of cell death [22]. The measurement was carried out within short time, because of the decrease in fluorescence intensity over time. Fluorescence bioimaging has strong advantages for in vivo tracing of altered biological processes and detection of specific molecule(s) in living zebrafish. However, owing to photo bleaching, fluorescence bioimaging of zebrafish showed there was not clear discrimination of the liver anatomically in this study. To confirm cell death in the liver, histopathology assessment was applied in this study. Cell death figures featured more frequently in DEN-treated liver tissues, showing general agreement for detection of cell death. However, owing to the small liver size, histopathology assessment has some limitation, resulting in large sample size. On the while, there were several cell deaths were existed in intestinal cells, irrespective of DEN treatment.
Formalin is one of the most common fixatives used for histology practices. In this study, zebrafish was fixed in 10% formalin and was transferred into cold ethanol for 24 hours. Without this step, it was fragile during tissue preparation, especially during sectioning (data not shown). A recent study reported that fixation with 10% formalin often resulted in shrinkages, creating large gaps between the liver and surrounding tissues [23]. Therefore, it seems it is important to select fixative(s) and fixation time according to the age of zebrafish.
Taken together, in vivo fluorescence bioimaging is considered to be a good method to detect cell death in the liver of zebrafish treated with DEN, when the outcome is confirmed with histopathological examination. The results of this study have given the possibility of using the zebrafish as a liver carcinogenesis model.







