Introduction
Antimicrobial feed additives have been widely used as a tool for enhancing growth performance in the swine industry [1, 2]. However, the emergence of antibioticresistant pathogens has raised public health concerns, to the extent that non-therapeutic antibiotics in feed additives were prohibited in Europe and South Korea [3–5]. Thus, there is an urgent need to develop effective and safe alternative feed additives, without the residual effects, to improve growth performance and immunity in farm animals [2, 6].
After the use of non-therapeutic antibiotics was prohibited in swine farms, researches have been widely conducted for developing the novel agents as feed additives to increase growth performance and immune responses in the swine industry [7–9]. The immunity of pigs directly affected susceptibility for various bacterial and viral infections in farm conditions. The increased resistance of antibiotics has been reported in swine farms raising public health concerns. Also, the use of antiviral materials was limited in swine farms due to their toxicity, high costs, and restricted effective spectrum for viruses [7–11].
Recently, the herbal mixture compound KIOM-C was found to improve growth performances and immune responses in porcine circovirus associated disease (PCVAD) infected weaning piglets under experimental conditions [12]. Moreover, KIOM-C evoked antiviral cytokines secretion against several viruses and inhibited virus replications in hosts [12–15]. However, the previous reports related to KIOM-C have been performed on only weaning piglets, evaluating non-specific immune responses after shortterm use of KIOM-C under experimental conditions.
The purpose of this study was to evaluate the effects of long-term use of commercialized KIOM-C on growth performance, nonspecific immune response, and specific humoral immune response against the foot-and-mouth disease virus (FMDV) in growing-to-finishing commercial pigs under farm conditions.
Materials and Methods
We used commercially available KIOM-C product (Vitabio Inc., Daejeon, Korea) in this study [12, 13, 15]. This product contains the mixtures of various herbal compounds including Zingiber officinale (Z. officinale), Scutellariae radix (S. radix), Platycodon grandiflorum (P. grandiflorum), Glycyrrhiza radix (G. radix), Paeoniae radix alba (P. radix alba), and Angelicae gigantis radix (A. gigantis radix).
All pigs were reared in a Korean commercial pig production system. The complex produces approximately 35,000 slaughter pigs from 1,500 crossbred sows (Landrace × Yorkshire) annually. At 10 weeks of age, pigs were moved into the growing-to-finishing complex, with slatted floor, where they remain until reaching approximately 110 kg in body weight. Windows and electrical ventilators were used for air ventilation and to maintain the temperature at approximately 19 ± 1℃. All animals are vaccinated against FMDV at 60 and 110 days old, using the commercially available FMDV vaccine (ARRIAH-VAC®, FGBI ARRIAH, Vladimir, Russia). The farrowing-toweaning complex has a history of Haemophilus parasuis, Streptococcus suis, and Mycoplasma hyopneumoniae infections. The growing-to-finishing unit was infected by pathogenic bacteria from a previous barn. In addition, porcine respiratory and reproductive syndrome virus (PRRSV) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) were also present.
Basal diets fed to pigs at each stage (weaners, growers, and finishers) were commercial concentrated feeds obtained from the market with the following specifications: 1) Weaner feed: ME 3,460 kcal/kg, crude protein 19.01%, crude fat 5.10%, crude fiber 3.39%, crude ash 5.19%, lysine 1.20%, calcium 0.79%, and phosphorus 0.55%; 2) Grower feed: ME 3,200 kcal/kg, crude protein 15.52%, crude fat 2.65%, crude fiber 4.01%, crude ash 4.76%, lysine 1.05%, calcium 0.85%, and phosphorus 0.45%; and 3) Finisher feed: ME 3,150 kcal/kg, crude protein 13.17%, crude fat 3.20%, crude fiber 3.18%, crude ash 3.91%, lysine 0.80%, calcium 0.58%, and phosphorus 0.40%. Feed and water were provided ad libitum.
A total of healthy 160 pigs (70 days of age) were selected from the weaner unit. The pigs were then divided into 2 groups (80 pigs per group) as follows: KIOM-C treated (KT) group and non-treated (NT) group. Each group, comprising of pigs of the same sex and body weight (p>0.05), was held in separate locations. Each group was separated into eight trial pens (ten pigs per pen) and pigs individuals could be identified by their ear-markings. The selected groups of weaner pigs were not fed performance enhancers, probiotics, antimicrobials, or acidifiers. After moving weaner pigs to their trial pens, the KT group was treated with KIOM-C at the level of 2 kg/tonne of basal feed from 70 days of age to slaughter age. KIOM-C was not added to the basal feed components of the NT group.
In order to estimate the average daily weight gain (ADWG) of each group, pigs were weighed three times at the start the growing stage (70 days of age), the start of finishing stage (119 days of age), and the end of the experiments (182 days of age). The age of pigs reached at slaughter weight (110 ± 5 kg) was recorded to estimate average slaughter ages. Quantities of feed intake per pen of pigs were recorded every week by calculating the difference in weights (kg) between the quantity of feed and residual feed, and then the average daily feed intake (ADFI) of each group was calculated. The feed conversion ratio (FCR) was calculated as the total feed intake divided by the total body weight gain of each group in each trial period.
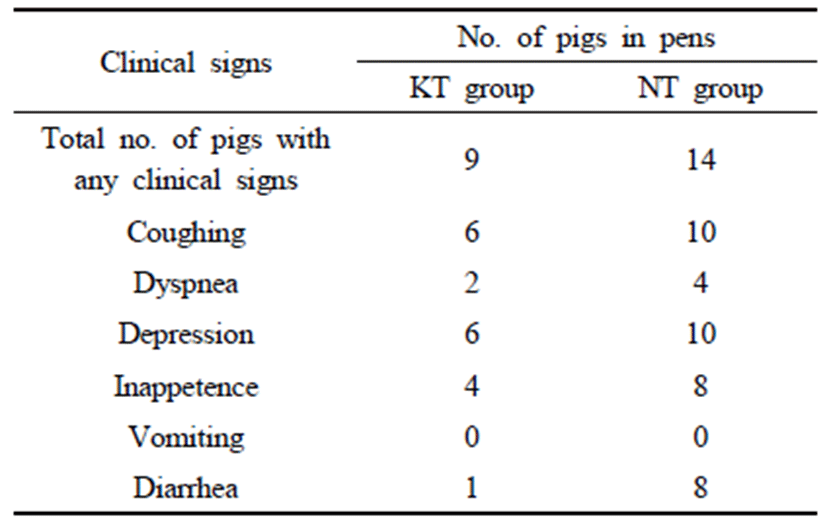
The clinical signs such as coughing, dyspnea, depression, inappetence, vomiting and diarrhea were monitored twice a day for all trial pigs by the farm technicians. If disease symptoms were detected, the affected pigs were allocated into a separate pen designed for sick pigs and treated by a swine veterinary expert. Deaths were recorded weekly by farm technicians throughout the trial period. We performed an autopsy for all deaths and examine the microorganism infection when suspected lesions were found. The blood of 30 pigs per group was collected at 70 days and 130 days of age, respectively.
Blood samples were collected from jugular veins in pigs. Serum was aseptically obtained from the supernatant of blood samples after centrifugation at 12,000 rpm for 20 min. Serum concentrations of porcine specific tumor necrosis factor-a (TNF-α) and interferon-γ (IFN-γ) were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Quantikine; R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer’s protocols. The serum IgA titers were determined by the Pig IgA ELISA Quantification Kit (Bethyl Laboratories, Montgomery, Texas, USA) following the manufacturer’s instructions. In order to assess the humoral immune responses specific for FMDV, anti-FMDV serotype O antibody levels of experimental animals were measured using the commercial ELISA Kit (PrioCHECK FMDV type O ELISA kit, Prionics AG, Schlieren-Zurich, Switzerland) as per manufacturer’s protocols. The percentage inhibition (PI) values were calculated by measuring the optical density (OD) at 450 nm, where PI values below 50% reflected an absence of anti-FMDV type O antibodies in a test serum.
Pens and individual pigs were regarded as an experimental unit for growth performance data and immune analysis, respectively. GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, USA) was used to analyze the data using a one-way ANOVA followed by a Tukey’s HSD post hoc analysis. The difference in the survival rates between the two groups was evaluated by two statistical methods including Kaplan-Meier method and log-rank test. Statistical significance was accepted at the level of p value<0.05.
Results
The growth performance parameters in groups of pigs were described in Table 1. During the entire trial period (70–182 days of ages), the ADWG and ADFI of the KT group was significantly higher than the NT group (p< 0.05), and the FCR of the KT group was significantly greater than the NT group (p<0.05). Compared with the NT group, the KT group had a significantly higher ADWG (p<0.05) at the growing stage. The ADFI of the KT group at the growing stage was significantly greater than the NT group (p<0.05); however, the FCR was similar in all groups during the growing stage (p>0.05). During the finishing stage, the KT group had a significantly higher ADWG and ADFI compared to the NT group (p<0.05). According to the FCR calculated for the finishing period, the KT group had a significantly better-feed utilization than that of the NT group (p<0.05). When average slaughter age was calculated for pigs reaching slaughter weight (110 ± 5 kg), there was a significant decrease in the time taken for pigs to reach slaughter weight in the KT group compared to the NT group (p<0.05).
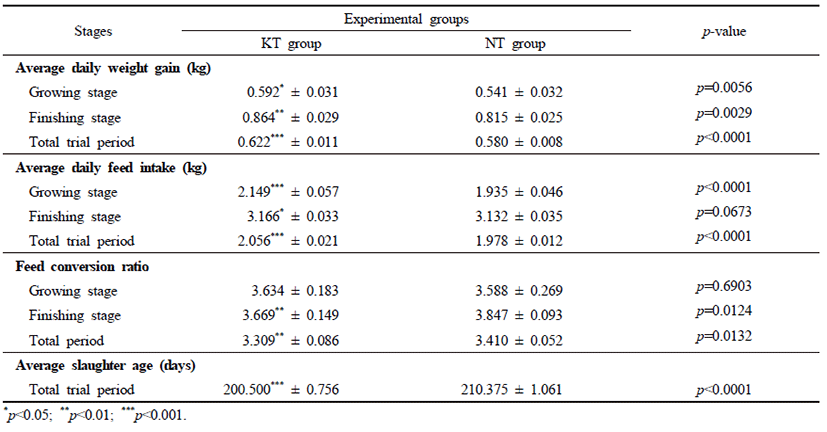
The survival rates in pigs of the NT and KT groups were evaluated throughout the trial periods. The survival rates of the KT group seemed to be higher than that of the NT group; however, this difference was not statistically significant (p>0.05) (Fig. 1). The concomitant infection of PRRSV and A. pleuropneumoniae was identified in pigs in the growing and finishing stages. Disease symptoms during experimental trials were evaluated for all pigs. NT pigs tended to represent various clinical symptoms more frequently compared to the KT group including coughing, dyspnea, depression, Inappetence and diarrhea (Table 2).
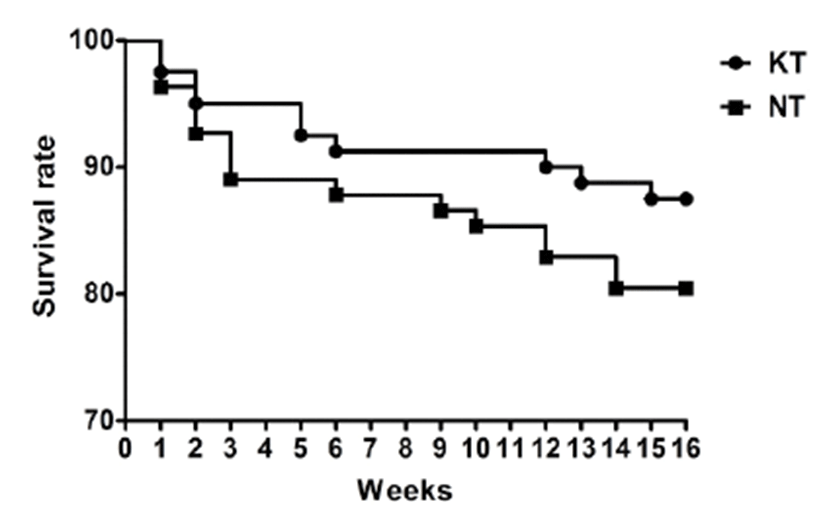
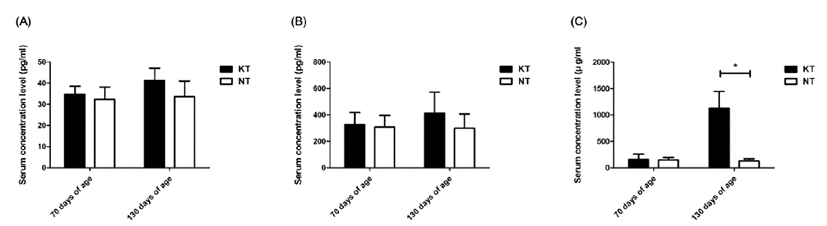
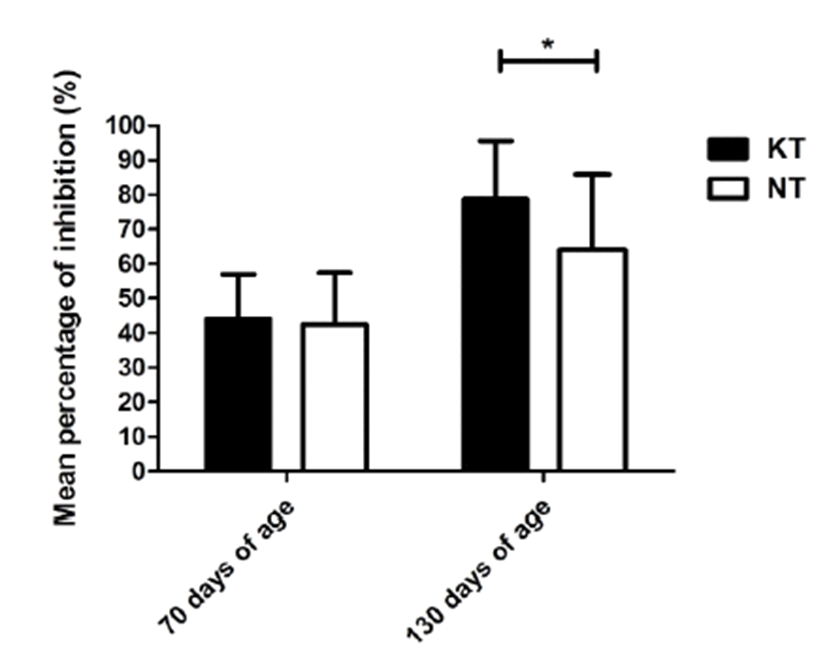
Concentrations of IFN-γ, TNF-α, and IgA in the experimental groups were successfully obtained in serum samples collected from pigs. Serum IFN-γ and TNF-α levels in the KT group increased slightly after treatment with KIOM-C; however, no significant difference was found between the KT and NT groups (p>0.05). Interestingly, the IgA level of the KT group at 130 days of age increased significantly, by more than 7 times, compared to the NT group (p<0.05) (Fig. 2). Additionally, the level of FMDV type O specific antibodies increased significantly in the KIOM-C supplemented group compared to the NT group (p<0.05) (Fig. 3). The percentages of antibody positive animals against the structural protein of serotype O after the second vaccination were 86.67% and 66.67% in the KT group and NT group, respectively.
Discussion
Feed additives are necessary for improving productivity in the swine industry. However, with the demands of consumers tending towards safe animal products, antimicrobial feed additives have been banned in Korea and Europe [4, 5]. In order to replace non-therapeutic dietary antimicrobial agents, researches on eco-friendly agents such as probiotics, organic acids, enzymes, and herbal plants have been conducted worldwide [1, 16–18]. In the present study, the clinical trial on the herbal feed additive, namely KIOM-C, developed in a previous study [12], was conducted in a growing-to-finishing pig production system in order to evaluate the effects on productivity and immune response.
The long-term application of KIOM-C on pigs between growing and finishing stages had a positive effect on ADWG, ADFI, and FCR decreasing the period of pigs to reach slaughter age. The KIOM-C treated groups also showed a tendency to have lower mortality and morbidity rates compared to NT group. The benefits of KIOM-C on growth performance may be due to nutritional effects, regulation of immune function, antimicrobial effects, and antioxidative actions [19]. KIOM-C contains herbal plants such as Z. officinale, S. radix, P. grandiflorum, G. radix, P. radix alba, and A. gigantis radix [12, 13, 15]. Z. officinale is known to have antimicrobial and anti-inflammatory effects, antioxidative action, and positive effects on growth promotion in broilers, although individual effects on pigs have not been verified [20, 21]. S. radix has both anti-inflammatory and antibacterial effects [22, 23]. P. grandiflorum activates B cell and macrophage responses, and improves intestinal health and immune responses of pigs, and when combined with G. Radix, has antiviral activity against rotavirus infection in piglets [24, 25]. Therefore, the combined effects of these herbal compounds could improve growth performance of the KIOM-C treated group in this study.
In a previous study, KIOM-C proved to increase protection against PCVAD infection in weaning piglets [12]. In this study, the levels of IFN-γ and TNF-α in KT group showed significant differences compared to those of NT groups. Interestingly, total serum IgA titers increased significantly in KT group. IgA is a major immunoglobulin of humoral immunity, which mainly contributes to protection against local pathogens in the mucosal surface in the form of secretory IgA (sIgA) [26, 27]. Serum IgA acts as a second line of defense by forming an immune complex with the aggressive pathogens and then interacting with receptors in the Fc region of IgA (FcaR) on the surface of the immune effector cells. This mechanism promotes antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis [27–30]. Thus, the substantial increase in serum IgA levels in this study signifies immune- stimulatory effects of KIOM-C, especially as mucosal and inflammatory immunity.
IgA might have the positive effects on growth performance (Table 1). IgA proved to form passive immunity in the intestinal environment of newborn piglets by secreting from the mother’s colostrum or milk through the gutmammary axis, and IgA synthesis increases temporarily postpartum in order to deliver maternal immunity to neonatal piglets [31, 32]. Therefore, further studies are necessary to determine the effects of KIOM-C on a sow’s lactogenic immunity and its protective effects against intestinal pathogens for neonatal piglets. High levels of serum IgA, however, could elicit the accumulation of FcRI-IgA complexes into the kidney and induce nephropathy in older humans [33, 34]. Although any evidence of disease derived from IgA nephropathy was not found in the present study, additional studies are necessary to determine the possibility of IgA nephropathy following the long-term use of KIOM-C.
FMDV infection, a highly contagious viral disease mainly occurring in cloven-hoofed animals, has resulted in economic losses in the swine industry by increased costs associated with vaccination and disease eradication policies [35]. Vaccination policies have been adopted worldwide to prevent FMD outbreaks, however, the vaccination of pigs generally induces poor humoral immune responses compared to those of cattle [36, 37]. This issue has prompted several approaches to enhance immune responses to vaccinations [38–40]. The results of the present study showed that KIOM-C supplementation in the diets of pigs elicited significantly higher anti-FMDV antibody levels and showed more than 80% seroconversion rates, which were an adequately positive rates for herd immunity, as mentioned in previous studies [37, 41, 42]. Therefore, these findings suggest that KIOM-C may be a potential immune-stimulator for enhancing the efficacy of FMDV vaccination, although further research should be performed to investigate the accurate mechanism study for enhancement of KIOM-C on humoral response.
In conclusion, KIOM-C compound had a positive effect on growth performance, an elevated activity on serum IgA levels and antibody response against FMD serotype O in growing-to-finishing pigs. Therefore, KIOM-C may have the potential to be applied as a feed additive to improve humoral immunity and productivity in commercial pigs. However, further research should be performed to clarify the underlying mechanisms associated with the positive effects of KIOM-C in this study and to establish the longterm safety of KIOM-C usage on pigs.







