Introduction
Monitoring the clinical disease status in progressive chronic kidney disease (CKD) is essential. In particular, persistent damage to the kidneys caused by symptoms such as hypertension and proteinuria is associated with prominent progressive CKD, temporally stable as subclinical CKD, indefinitely subclinical CKD stable as subclinical CKD [1]. Blood pressure (BP) measurement and urinalysis, including testing for proteinuria, are an important part of a clinical evaluation of dogs and cats that show signs of kidney disease.
Hypertension in cats is closely associated with CKD. Azotemia was also found in 74% of cats with hypertension, and the incidence of hypertension was found to be 19−65% in CKD cats [2−4]. In human medicine, sodium intake, water retention, activity of the renin angiotensin aldosterone system (RAAS) and of sympathetic nerves, structural changes of the arterioles, endothelial dysfunction, oxidative stress, and genetic factors play important roles in the pathogenesis of hypertension associated with CKD [5]. Little is known about the mechanism of hypertension in cats with CKD, but limited changes in the RAAS or BP after the use of angiotensin-converting enzyme inhibitors (ACEi) suggest that RAAS activation may not be a major contributor [6, 7]. Telmisartan, an angiotensin receptor blocker (ARB), is considered effective against hypertension in cats [8]. However, to our knowledge, there is only one case report showing the efficacy of telmisartan against systemic hypertension in cats with symptoms of hypertension that do not respond to benazepril [9]. Further studies of the efficacy of telmisartan in controlling blood pressure are warranted.
The causes of proteinuria are very diverse. Sometimes, proteinuria appears temporarily due to high temperature, stress, seizures, venous congestion, fever, or extreme exercise as a functional or physiological proteinuria that is not a direct cause of kidney disease and shows no severe degree of urinary protein levels [10]. In addition, some studies on functional proteinuria in cats and dogs have reported a clearer relationship of stress to proteinuria than exercise [11]. In several human studies, proteinuria was found to be correlated with the progression of CKD, as an index factor of heart disease and diabetic nephropathy [12−15]. In several studies of cats and dogs, proteinuria has been reported to be highly correlated with a reduced rate of survival, regardless of the patient’s azotemia status [1, 16−24]. Therefore, it is necessary to control the proteinuria to improve the survival rate of patients with proteinuria. [1, 24]. In some studies, the effectiveness of kidney protection along with decreased levels of proteinuria was proven after treatment with ACEi in dogs and cats with distinct proteinuria [25, 26]. In addition, a study on cats showed that urine protein to creatinine ratio (UP/C) was significantly improved in the telmisartan treated group compared to the control group treated with benazepril [27].
The aim of this study was to compare over time the changes in various indicators, including hypertension and proteinuria, before and after the administration of telmisartan in cats with CKD.
Materials and Methods
The study was conducted on cat patients of CKD with no other diseases. A total of 40 cats (22 spayed females and 18 castrated males, weighing 2.0−7.9 kg) aged 1−19 years (mean 9.5 ± 4.6), were enrolled.
Cats examined in this study were selected using several criteria. Cats with symptoms of diseases other than kidney failure, such as heart disease or pancreatitis, were not included. Cats who were clinically evaluated as having CKD and who met one or more of the following criteria were included: according to the International Renal Interest Society stages [28], patients with CKD at 2−4/4 grades who have serum creatinine concentrations exceeding 1.6 mg/dL, UP/C ratio of >0.2 [1], and hypertensive systolic BP of >150 mmHg on Doppler [8, 29]. In addition, the owner should be present, and individuals weighing less than 2 kg were excluded.
Before and after telmisartan (Semintra® oral solution, Boehringer Ingelheim, Mexico) administration (1 mg/kg, PO, SID), patients were followed 4 times at 30-day intervals for a total of 90 days. Body weight, serum chemistry (IDEXX Catalyst Dx TM Analyzer, IDEXX Laboratories, USA), total cell blood count (IDEXX Procyte Dx TM Hematology Analyzer, IDEXX Laboratories, USA), urine specific gravity, urine pH, and UP/C ratio (IDEXX Catalyst Dx TM Analyzer, IDEXX Laboratories, USA) tests were performed at each visit over the course of the follow up period.
BP was measured using a Doppler device (Parks Model 811-B, Parks Medical Electronics, USA) according to the consensus guidelines of the International Society of Feline Medicine [8]. Before being examined, cats rested calmly for 5−10 minutes in the examination room. The placement and size of the inflatable cuffs were determined by measuring the forelimbs with the short length of the cuff to be 30%−40% of the length of the forelimb. The characteristic ultrasonic sound of the Doppler devices can cause tension in cats, and headphones were used. After ultrasonic gel was applied to the leg region to be measured and the transducer closely attached, the sphygmomanometer was used to inflate the cuff to a pressure of 20−40 mmHg until no Doppler ultrasound signals generated by the red blood cells could be heard. If no sound was heard, the sphygmomanometer was deflated until the sound again could be heard, and at the first moment of Doppler ultrasound detection systolic blood pressure (SBP) was measured.
Statistical analyses were performed using commercially available statistical software (Microsoft Office Excel 2016 for Mac). Continuous variables are presented as mean ± standard deviation. Differences in the various indices that change with time before and after administration of telmisartan were assessed using one-way ANOVA. In all comparisons, p<0.05 was considered statistically significant, unless stated otherwise.
Results
The following breeds of cats were included in this study: Abyssinian (1/40, 2.5%), American Shorthair (1/40, 2.5%), British Shorthair (1/40, 2.5%), Domestic Short Hair (12/40, 30.0%), Himalayan (1/40, 2.5%), Persian (3/40, 7.5%), Russian Blue (5/40, 12.5%), Scottish Fold (1/40, 2.5%), Siamese (8/40, 20.0%), Sphynx (1/40, 2.5%), Turkish Angora (6/40, 15.0%). Domestic Short Hair, Siamese and Turkish Angora breed were overrepresented in this study.
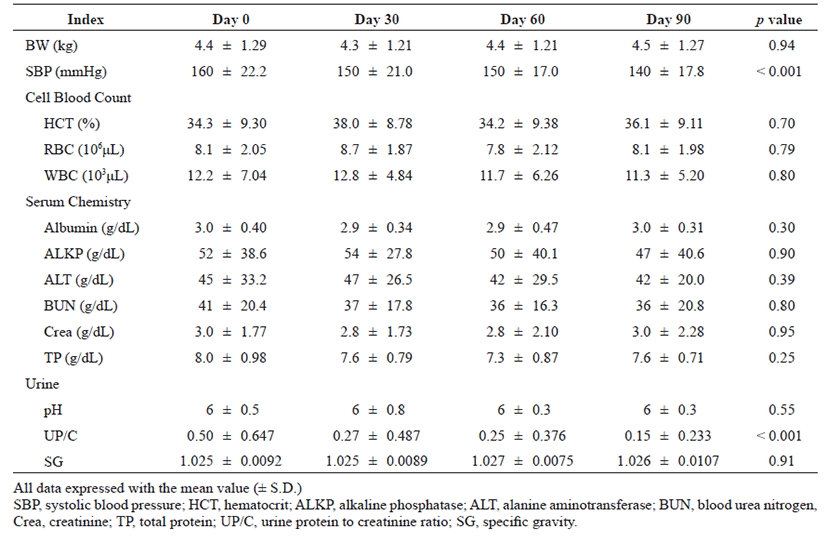
The cell blood count results showed no significant changes in red blood cells and white blood cells. Serum chemistry analysis showed an elevation of creatinine and blood urea nitrogen (BUN), signs of worsening of kidney disease; however, the changes were not statistically significant. In addition, there were no statistically significant changes in alanine aminotransferase (ALT), alkaline phosphatase (ALP), and BUN as predictors of liver function. No significant changes in the hematocrit or total protein (TP) values related to dehydration were found. As a result of urinalysis, changes in neither urine pH nor urine specific gravity (USG) were found to be statistically significant. However, although not statistically significant, body weight was slightly increased after treatment (p=0.94).
Decrease in BP (p<0.001) and UP/C (p<0.001) were found to be statistically significant over time after the administration of telmisartan. BP and the UP/C ratio were 160 ± 22.2 and 0.50 ± 0.647 before telmisartan administration (Day 0), 150 ± 21.0 and 0.27 ± 0.487 on the 30th day (Day 30), 150 ± 17.0 and 0.25 ± 0.376 on the 60th day (Day 60), and 140 ± 17.8 and 0.15 ± 0.233 on the 90th day (Day 90) after administration, respectively. BP and UP/C were statistically significantly lower in cats with CKD over time at each time point from Day 0 to Day 90 at 30 day intervals (Fig. 1, A and B). Especially after 90 days of telmisartan administration, the improvement of BP and UP/C were estimated to be about 20 mmHg and 0.35, respectively. Differences in the various indices that changed with time before and after drug administration in this study population were summarized in Table 1.
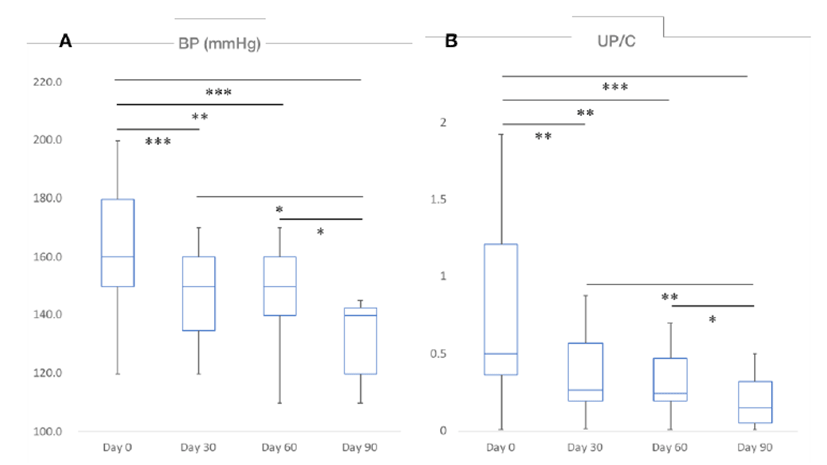
|
Discussion
Our study revealed a clear and gradual decrease in BP and UP/C ratio in cats with CKD after administration of telmisartan. The kidney glomeruli can filter out circulating protein due to its size, so that it is not excreted and remains in the body. Even if some fine proteins pass through the kidney glomeruli, they will be reabsorbed in the tubules and proteins will not show up in the urine of healthy cats. Therefore, the appearance of protein in the urine is likely to be a sign of disease of the kidneys, including in the glomeruli and tubules. However, when kidney failure occurs, the glomerular filtration rate of the kidneys decreases, resulting in symptoms such as polyuria (PU) and polydipsia (PD). To compensate for this, the RAAS is activated and vasocontraction of efferent arterioles results in elevated blood pressure, increased pressure in the glomeruli, and leakage of proteins due to glomerular injury. ACEi have been traditionally used to correct the RAAS that activity causes such problems. Recently, ARB agents such as telmisartan have been attracting attention; however, to date, there has not been much research available on ARB in veterinary practice.
ACEi could cause vasodilation of efferent arterioles, possibly resulting in the elevation of creatinine; however, our study with telmisartan did not result in azotemia, including that caused by creatinine. Angiotensin II (AT-II), induced by activation of RAAS, activates several receptors. Among these, angiotensin 1 (AT1) receptors have a negative effect on vasoconstriction, sympathetic activation, cell proliferation, aldosterone release, and renal sodium reabsorption, and angiotensin 2 (AT2) receptors have a positive effect on vasodilation and apoptosis as well as an antiproliferative action. ACEi, such as benazepril or enalapril, block ACE, which initiates the process of converting angiotensin I to angiotensin II, inhibiting RAAS activation. However, ACEi do not completely block RAAS due to the escape pathway that avoids the process of converting AT-I to AT-II, so that some AT1 receptors are still activated. In addition, ACEi block AT2 receptors; therefore, even positive effects are interrupted. ARBs such as telmisartan selectively block the AT1 receptor and do not affect the AT2 receptor, and thus have a mechanism capable of effectively blocking the activity of RAAS. [30]. In human studies, telmisartan has been reported to have a renal clearance of 1%, liver clearance of 99%, a biological half-life of 24 hours, protein binding of 99% or more, and bioavailability of 42%−58%, but has no effect on food [31].
ALT is a leaking enzyme in liver damage, albumin is synthesized and stored in the liver, and ALP plays an essential role in liver metabolism and skeleton development. Because BUN is a byproduct of protein degradation and is produced by the urea cycle in the liver, BUN concentration decreases in cases of liver damage. Although telmisartan may have a high burden on liver clearance, our study did not reveal statistical significance as judged by hepatic injury evaluated by serologic tests of liver.
Repeated tests for urinary albumin in cats with persistent proteinuria (UP/C>0.5) did not detect albumin [32]. The method of measuring the total amount of protein by collecting urine for 24 hours and measuring the UP/C by collecting a single sample at one time period, showed a good correlation [33, 34]. In this respect, the gold standard test for the measurement of proteinuria is to measure UP/C [35], and our study also used UP/C measurement to evaluate proteinuria. In this study, all urine specimens were collected by ultrasound guided cystocentesis. Therefore, there was no gross hematuria reaction and although urine chemistry showed occult blood reactions, this occult blood had no effect on the measurement of proteinuria [36]. In our study, the UP/C ratio improved with the passage of time after telmisartan application, and urine pH and USG did not change. This suggests that telmisartan does not cause chemical changes in urine, but has a vasodilatory effect. In conclusion, the oral administration of telmisartan to cats with CKD is effective in improving blood pressure and proteinuria, which has a positive effect on long-term survival in cats with CKD.







