Introduction
Myxomatous mitral valve disease (MMVD) is the most common cardiac disease in dogs and is characterized by chronic myxomatous mitral valvular degeneration resulting in mitral regurgitation (MR) [1−4]. Transthoracic echocardiography is the diagnostic method of choice to identify mitral valve lesions and determine the severity of MR. The technique is also useful to assess the degree of cardiac remodeling and myocardial dysfunction as well as changes in left ventricular filling pressures and pulmonary arterial pressure [1, 5−9].
Echocardiography is one of the most useful diagnostic techniques to differentiate between the types of heart disease. In addition, mitral valve lesions can also be identified using two-dimensional and M-mode echocardiography [1, 10, 11]. One study found that the severity of mitral valve prolapse (MVP) significantly correlated with that of MR [12]. Color-flow doppler imaging (CDI) is useful to detect and evaluate the degree of MR in dogs with MMVD. Studies have also reported correlation between the degree of left atrium (LA) dilation, assessed by the LA to aorta (LA/Ao) ratio, and the severity of heart failure [13-15]. Left ventricle (LV) volume overload may commonly occur with increasing severity of MR, due to volume retention, remodeling, and elevations in LA and pulmonary venous pressures [16]. Therefore, an increase in the end-diastolic volume (EDV) may correlate with the severity of MR in dogs with MMVD [17]. The common indices of left ventricular systolic myocardial function such as ejection fraction (EF) and fractional shortening (FS) are generally elevated in dogs with MMVD due to increased preload, reduced afterload, and hyperdynamic ventricular contraction [18]. Systolic myocardial dysfunction may also be observed in dogs with advanced stages of MMVD [5, 7, 19].
Spectral doppler methods, including pulsed wave Doppler (PWD) and continuous-wave Doppler (CWD) are useful to determine regurgitant jet, trans-mitral flow in dogs with MMVD. Diastolic function and left ventricular filling pressures can be assessed using the PWD method [20], while CWD is useful to assess the severity of MR and thus provides information on LA pressure, preload, and systemic arterial pressure [5].
The present study evaluated the conventional echocardiographic indices to assess the severity and progression of MMVD in dogs.
Materials & Methods
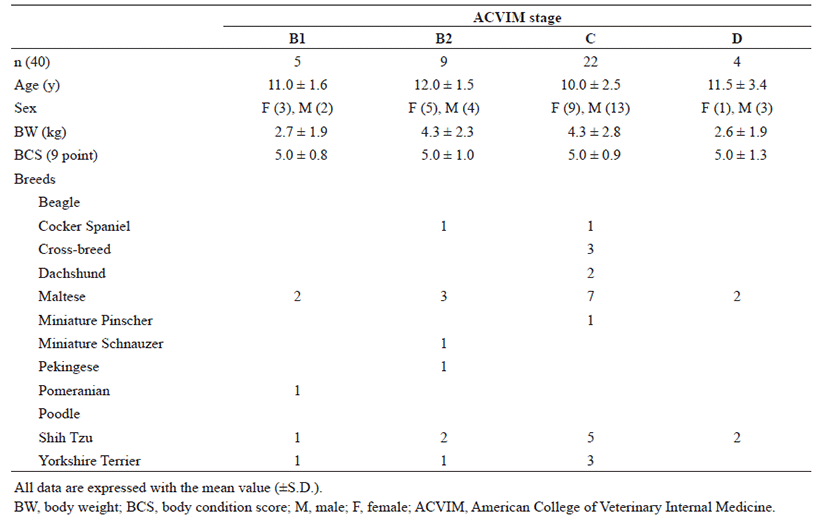
Forty small breed dogs (weight, 2.3−13.2 kg) aged between 8−17 years with MMVD were included. All dogs with MMVD had been presented for a cardiology consultation due to previous identification of a heart murmur, presence of clinical signs indicating cardiovascular disorder, including cough and exercise intolerance, or both. The consent of the owner for inclusion of each dog was obtained before enrolment in the study. All dogs underwent physical examination, echocardiography, complete blood count, and chemistry panel investigations. Dogs with other clinically relevant systemic diseases, such as renal failure and hypertension were excluded. Diagnosis was made based on clinical signs, chest radiography data, and echocardiographic findings, according to the standard guidelines for the diagnosis of MMVD in dogs [21]. The dogs with MMVD were classified based on the criteria proposed by the American College of Veterinary Internal Medicine (ACVIM) for the functional classification of heart failure [21]. Dogs with symptomatic MMVD were prescribed medications such as enalapril, furosemide, spironolactone, pimobendan, and amlodipine depending upon the severity of the condition.
Echocardiographic examinations were conducted in accordance with recommended standards for dogs. Mmode, Doppler, and 2D echocardiography (Acuson X-300, Siemens, Mountain view, CA) were performed in left and right lateral recumbency. 2D echocardiography was used to measure LA and Ao diameter from 2D short axis at the level of the aortic valve. These measurements were used to determine LA/Ao ratio and left ventricular internal dimension in diastole (LVIDd) to aorta (LVIDd/Ao) ratio. M-mode echocardiography was used to measure left ventricular internal dimension in systole (LVIDs) and LVIDd from 2D short axis at the level of the papillary muscle. EDV was calculated using the following formula [7 × (LVIDd) 3 / (2.4 + LVIDd)] and end-systolic volume (ESV) was calculated as [7 × (LVIDs) 3 / (2.4 + LVIDs)]. End diastolic volume index (EDVI) was calculated as follows: EDV was divided by the body surface area (BSA) that is calculated by body weight (BW, kg). End systolic volume index (ESVI) was calculated by dividing the ESV by BSA. BSA of each dog was calculated using the formula: (10.1 × BW0.67) / 100. %FS was calculated using the formula [(LVIDd−LVIDs) / LVIDd] × 100 and %EF was calculated as [(EDV−ESV) / EDV] × 100. Continuous and pulse wave Doppler echocardiography was used to measure MR velocity, E-peak velocity and A-peak (A, late ventricular diastolic filling wave) velocity from apical four chamber view. These measurements were used to determine the E-peak velocity to A-peak velocity (E/A) ratio.
Statistical analyses were performed using commercially available statistical software (SPSS 12.0). Continuous variables are presented as mean ± standard deviation (SD). Differences in echocardiographic indices among groups were evaluated using one way ANOVA. Pearson’s correlation of bivariate correlation analysis was used to test the strength of association between heart failure stage as ACVIM classification and echocardiographic indices used for evaluation of the severity. In all comparisons, a probability value of p<0.05 was considered statistically significant, unless stated otherwise.
Results
Forty dogs (22 females and 18 males) were enrolled in this study. Demographic characteristics of the study population are summarized in Table 1. Dogs with remodeled asymptomatic heart disease due to MMVD (B2 stage, 12.0 ± 1.5) were slightly older than any other groups of dogs (Table 1). In addition, dogs with remodeled asymptomatic heart disease (B2 stage, 4.3 ± 2.3) and symptomatic heart failure due to MMVD (C stage, 4.3 ± 2.8) were slightly heavier than dogs in any other groups (Table 1).
The breeds of dogs included in this study are as follows (Table 1): Cocker Spaniel (2/40; 5.0%), Cross breed (2/40; 5.0%), Dachshund (2/40; 5.0%), Maltese (14/40; 35.0%), Miniature Pinscher (1/40; 2.5%), Miniature Schnauzer (1/40; 2.5%), Pekingese (1/40; 2.5%), Pomeranian (1/40; 2.5%), Shih Tzu (10/40; 25.0%), and Yorkshire Terrier (6/40; 15.0%). Maltese and Shih Tzu were the most common breeds of dogs included in this study. Maltese (2/5; 40.0%), Pomeranian (1/5; 20.0%), Shih Tzu (1/5; 20.0%), and Yorkshire Terrier (1/5; 20.0%) belonged to the B1 stage as per ACVIM, while Cocker Spaniel (1/9; 11.1%), Maltese (3/9; 33.3%), Miniature Schnauzer (1/9; 11.1%), Pekingese (1/9; 11.1%), Shih Tzu (2/9; 22.2%), and Yorkshire Terrier (1/9; 11.1%) were in the B2 stage. Cocker Spaniel (1/22; 4.5%), Cross breed (3/22; 13.6%), Dachshund (2/22; 9.1%), Maltese (7/22; 31.8%), Miniature Pinscher (1/22; 4.5%), Shih Tzu (5/22; 22.7%), and Yorkshire Terrier (3/22; 13.6%) were in ACVIM C stage. In addition, Maltese (2/4; 550.0%) and Shih Tzu (2/4; 50.0%) belonged to ACVIM D stage. Maltese and Shih Tzu breed were overrepresented in all groups.
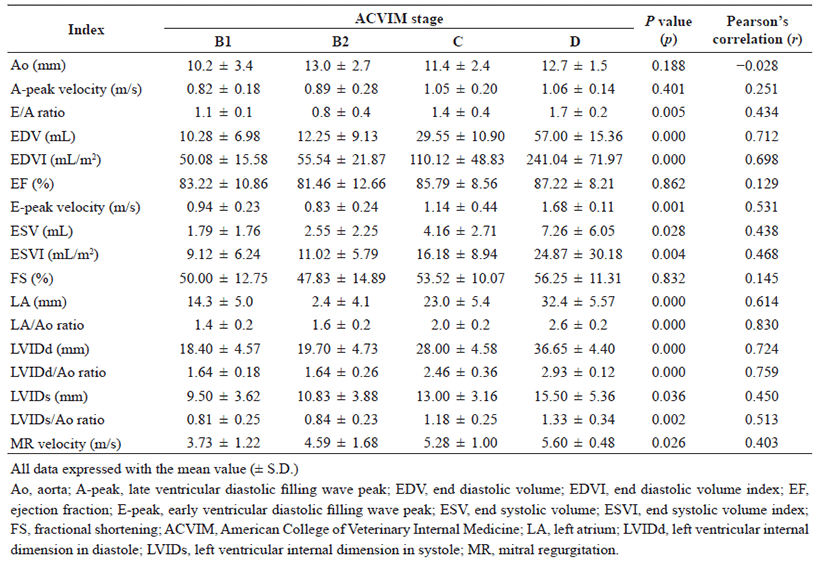
The conventional echocardiographic indices in the included study population are summarized in Table 2. In the comparison of conventional echocardiography indices in dogs with different stages of heart failure with MMVD, significant differences were observed in E/A ratio (p=0.005), EDV (p<0.001), EDVI (p<0.001), E-peak velocity (p=0.001), ESV (p=0.028), ESVI (p=0.004), LA (p<0.001), LA/Ao ratio (p<0.001), LVIDd (p<0.001), LVIDd/Ao ratio (p<0.001), LVIDs (p=0.036), LVIDs/Ao ratio (p=0.002), and MR velocity (p=0.026). In addition, distinct correlations were found in EDV (r=0.712), LA/ Ao ration (r=0.830), LVIDd (r=0.724), and LVIDd/Ao ratio (r=0.759).
Discussion
Several prognostic indicators have been identified using echocardiography and biomarkers in dogs with MMVD [22]. The ESVI, LA/Ao ratio, E-peak velocity, EDVI, and bilateral mitral valve leaflet engagement are established echocardiographic indices in dogs with MMVD [23]. In this study, the above-mentioned echocardiographic indices correlated with the severity of heart failure (i.e. progression of MMVD).
The LA/Ao ratio is generally indicated to the degree of LA dilation and shows satisfactory correlation with the stage of heart failure stage [10]. One study reported significant correlation of the LA/Ao ratio exceeding 1.7 with survival time [23]. Therefore, the LA/Ao ratio increases significantly with worsening of MMVD. In the present study, the LA/Ao ratio correlated with the severity of heart failure (Fig. 1A).
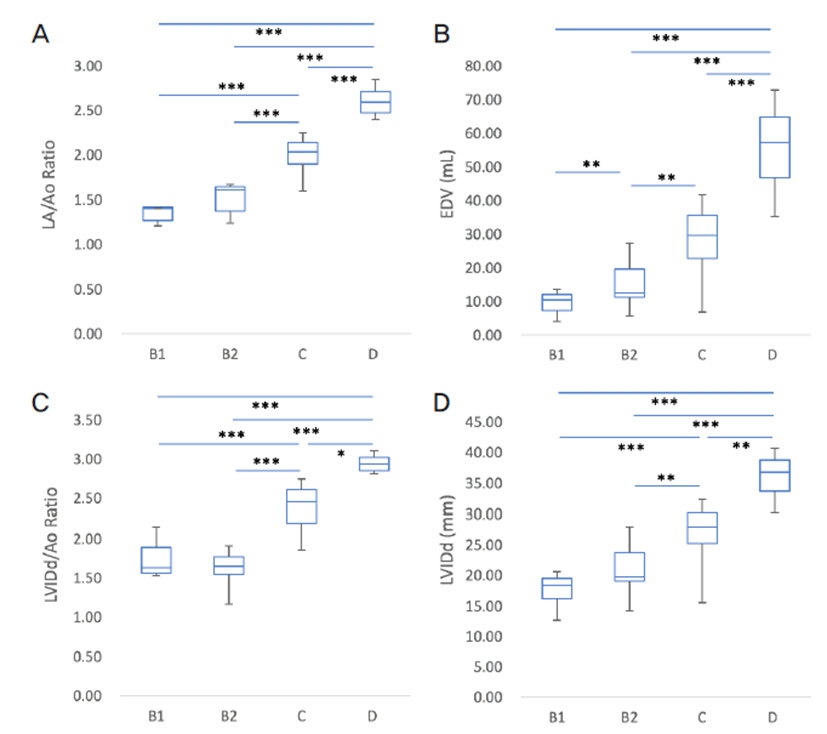
LVIDd and LVIDs indicate the degree of LV dilation by LV volume/pressure overload and have found to correlate with the severity of MMVD in dogs [17, 24]. In the present study, LVIDd index was significantly different and distinct correlation in dogs with different stages of heart failure with MMVD (Fig. 1D). As the reference range for LVIDd and LVIDs in dogs differed depending upon the breed, the LVIDd/Ao and LVIDs/Ao ratio may be a more favorable indicator for evaluation of the degree of LV dilation [20]. As expected, the LVIDd/Ao and LVIDs/Ao ratio were significantly different in dogs with different stages of heart failure and correlated with the severity of MMVD in the included study population (Fig. 1C). The EDVI is the index that is obtained by dividing the EDV by the BSA of the animal. Increase in EDV is affected by left ventricular volume overload, which has been reported to indirectly depict the severity of MMVD [5, 25]. In the present study, EDV was significantly different with the severity of heart failure with MMVD (Fig. 1B). However, the volume of the LA and LV do not completely reflect the pressure of the chambers because the chamber pressure may be affected by the compliance of the chamber wall and preload [26−30].
The EF and FS are the most common echocardiographic indices for representing LV systolic function. Several factors including preload, afterload, and left ventricular contractility can affect the measures of EF and FS [31]. Although lower FS and EF suggested poor preload, increased afterload, or decreased left ventricular contractility [31−33], the measures tended to increase with an escalation in the ACVIM class, because significant MR could lead to an increase in preload and decrease in afterload [7]. As reported in other studies, the present study also observed an increase in FS and EF in dogs with advanced stage of heart failure.
The trans-mitral flow velocities, including E and A, indicate myocardial diastolic function and left ventricular filling pressure [20]. Although several factors including age, heart rate, preload, myocardial relaxation, chamber compliance, and volume can influence trans-mitral flow velocities [28, 29, 34−37], some studies observed elevation in the values with progression of MMVD in dogs [38−40]. Generally, the E-peak velocity exceeding 1.5 m/s strongly indicates severe LA volume overload causing marked elevation of LA pressure and LV filling pressure [5]. In the present study, the E-peak velocity was significantly different in dogs with different stages of heart failure and correlated with the severity of MMVD.
The A-peak velocity is commonly lower than E-peak [41]. Higher A-peak velocity when compared to E-peak (so-called E/A reversal) suggests impaired myocardial relaxation. In addition, increased A-peak velocity indicates LA pressure overload. In the present study, the A-peak velocity and E/A ratio were not significantly different in dogs with different stage of heart failure, suggesting that myocardial diastolic function might not be affected by the progression of MMVD. However, several studies have reported diastolic dysfunction in dogs with advanced stage of heart failure [39, 42, 43].
MR velocity indicates the pressure gradient from LV to LA at systole and commonly ranges between 5−6 m/s [1, 5]. MR velocity can be influenced by several innate factors (e.g. loading condition, systolic blood pressure and LV contractibilty); therefore, the measure does not generally correlate with the severity of MMVD in dogs [24]. However, recent studies reported that MR velocity could be significantly dropped when dogs faced with mortality [20, 24]. However, unlike other studies, the MR velocity in the present study was significantly different in dogs with different stage of heart failure and was the highest in dogs in the ACVIM D stage, suggesting a strong correlation with the severity of MMVD.
The present study is associated with several limitations. First, the sample size was small to obtain results with definitive statistical significance. Second, several echocardiographic parameters may be influenced by hemodynamic changes by amounts of MR and systolic blood pressure [5, 18, 20]. Those two parameters can be affected by the excitement during the echocardiography, although echocardiography was performed in calm conditions with minimal restraint. Third, there was no wide-ranging deviation in the body weight of the included dogs. Echocardiographic indices such as EDV and LVIDd may vary significantly depending on the body weight of the animal. EDV and LVIDd showed favorable significance and correlation with the severity of heart disease in the present study due to minor deviation in the weights of the included animals. Finally, medications can affect cardiac contractility and loading conditions and thus may influence the echocardiographic indices. Many dogs in advanced stages of heart failure were under cardiac medication, suggesting that the echocardiographic indices could have been different, if medications were withheld. Therefore, further studies are warranted to evaluate the influence of cardiac medications on the echocardiographic indices.
In conclusion, the present study observed that the known conventional echocardiographic indices, including EDV, LA/Ao ratio, LVIDd dimension, and LVIDd/ Ao ratio correlated with the severity of MMVD in point of significant differences and distinct correlations.