Introduction
Arthritis shows biochemical and structural changes in cartilage and bony structures [1]. Studies on osteoarthritis (OA) have shown that chondrocytes in cartilage exhibit morphologic changes of apoptosis [2], and that apoptosis and proteoglycan depletion are anatomically linked [3]. Work on rheumatoid arthritis (RA) has suggested that chondrocytes might participate in degrading its own matrix by releasing autocrine-paracrine factors [4]. Research also points to the loss of differentiated phenotype of articular chondrocytes (known as dedifferentiation) as an important factor in the progression of arthritis [1]. In addition to apoptosis and dedifferentiation, higher amounts of reactive oxygen species (ROS) from inflammatory cells also play a role in the cartilage degradation process that takes place in the pathogenesis of arthritis [5].
Nitric oxide (NO) has been implicated as a biological mediator in degenerative diseases of cartilage, such as OA and RA. Research in this area revealed that excessive production of NO inhibits matrix synthesis and promotes its degradation, via increased expression of inducible NOS (iNOS) and cyclooxygenase-2 (COX-2), associated with increased expression of pro-inflammatory cytokines, particularly interleukin-1 [6]. In other research, it was determined that NO might play a role in apoptosis of chondrocytes, and that chondrocyte-derived apoptotic bodies expressed functional properties that might contribute to the pathologic cartilage calcification observed in OA [7]. A previous report showed that sodium nitroprusside (SNP) treatment as NO donor induced apoptosis and dedifferentiation in primary cultured articular chondrocytes [8].
Prednisolone is a steroid medication used to treat a wide range of inflammatory and autoimmune conditions, and it is a widely used to treat arthritis [9]. It has been shown to effectively slow the rate of joint destruction and substantially reduce the rate of RA progression [10]. It has also shown the potential to decrease trabecular bone mass and strength by reducing bone resorption and bone formation defect in adjuvant-induced arthritic rats [11]. However, the cellular mechanism by which prednisolone acts on cartilage degeneration and apoptosis of chondrocytes is largely unknown. The purpose of this study was to investigate the effect of prednisolone in SNP-treated rabbit articular chondrocytes.
Materials and Methods
Articular chondrocytes were isolated by enzymatic digestion from cartilage slices of 2-week-old three New Zealand white rabbits. All the chondrocytes from each animal were used for individual experiments. In brief, the cartilage slices were dissociated by 0.2% collagenase type II (381 U/mg solid; Sigma-Aldrich, St. Louis, MO) in Dulbecco’s Modified Eagle's Medium (DMEM) (Gibco-BRL, Gaithersburg, MD) for 6 h. Individual cells were suspended in DMEM supplemented with 10% (v/v) fetal bovine calf serum, 50 g/ml streptomycin, and 50 units/ml penicillin. Cells were plated onto culture dishes at a density of 5 × 104 /cm2. The medium was changed every 2 days after seeding, and cells reached confluence in approximately 5 days. This study was approved by the animal ethics committee of Kongju National University according to the Animal Protection Act.
The MTT assay was used to examine the proliferation of chondrocytes. Cells were seeded onto 96-well plates at a density of 2 × 104 /well for 24 h and then treated with SNP (298 μg/ml) alone or SNP plus prednisolone (0.05- 50 μg/ml). A 10 μl aliquot of MTT reagent 1 was added to each well. The plate was incubated for 4 h at 37°C until purple formazan crystals developed. 100 μl of MTT reagent 2 was added to each well. The absorbance was read at 600 nm with a spectrophotometer in four wells per treatment group after an overnight incubation.
Cell lysates were prepared by extracting proteins using a buffer containing 50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, and protease inhibitors (10 g/ml leupeptin, 10 g/ml pepstatin A, 10 g/ml aprotinin, 1 mM 4-[2-aminoethyl] benzenesulfonyl fluoride, and 1 mM NaF and 1 mM Na- 3VO4 as phosphatase inhibitors). The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes. Non-specific sites were blocked with Tris-buffered saline containing 3% non-fat dry milk and the membrane was incubated with the following antibodies: type II collagen (Santa Cruz Biotechnology Inc., Santa Cruz, CA), COX-2 (Cayman Chemical, Ann Arbor, MI) or pERK (Cell signaling, Beverly, MA), and a peroxidase-conjugated secondary antibody. The blots were washed and protein visualized using the enhanced chemoluminescence method. Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was used as internal standard.
Chondrocytes were fixed with 3.5% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. The cells were treated with 0.1% Triton X-100 and were blocked and 5% fetal calf serum in PBS for 30 min. The fixed cells were washed and incubated for 1 h with a type II collagen antibody. The cells were washed, incubated with fluorescein-conjugated secondary antibodies for 30 min, and observed by a fluorescence microscope.
Results
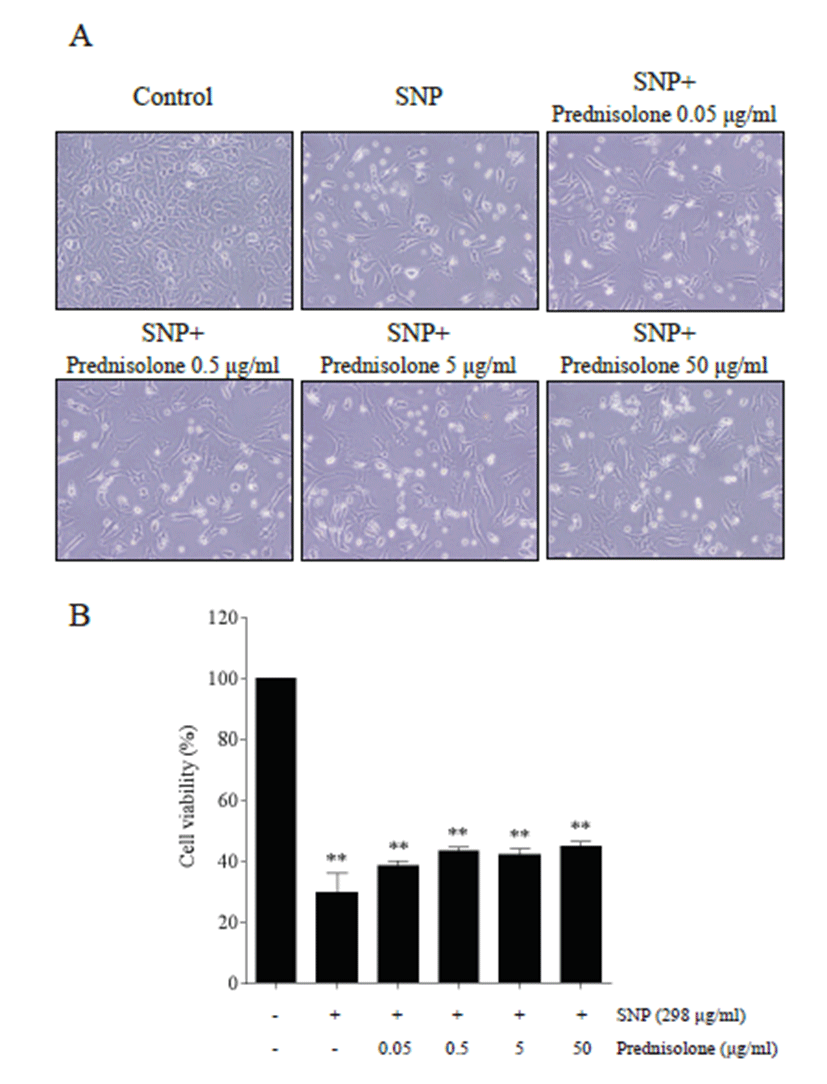
Various doses of prednisolone were used to assess the anti-apoptotic effect of prednisolone in SNP-treated cells. Cell morphology indicated that SNP treatment induced cytotoxicity, and that SNP-induced cytotoxicity was inhibited by prednisolone treatment (Fig. 1A). MTT assay showed that SNP treatment decreased significantly the level of cell viability compared with that of control (p <0.01), and that prednisolone treatment decreased SNPinduced cytotoxicity (Fig. 1B).
Western blot analysis showed that SNP and prednisolone treatment modulated type II collagen and COX-2 expression in rabbit articular chondrocytes (Fig. 2A).
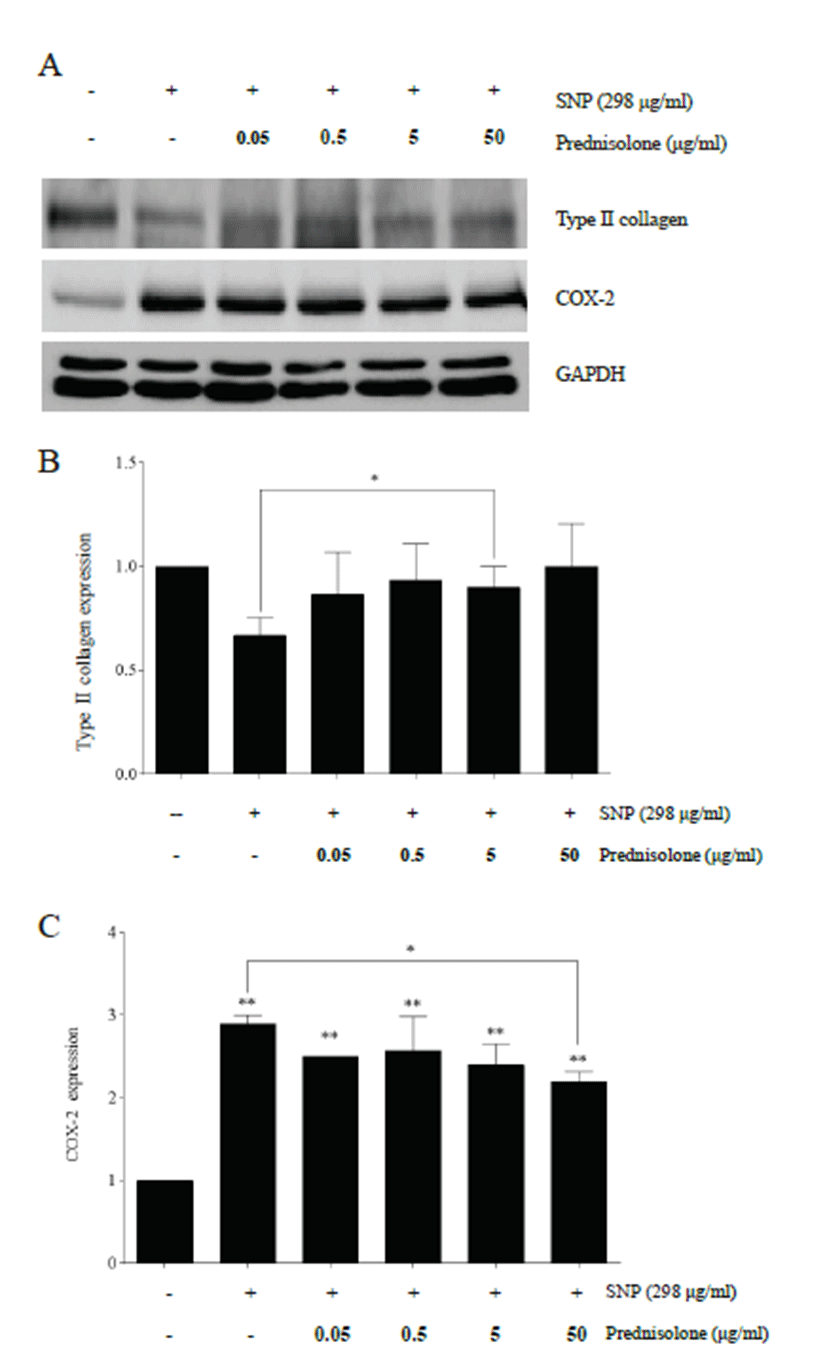
SNP treatment decreased type II collagen compared with control chondrocytes, and prednisolone treatment recovered the down-regulated expression of type II collagen induced by SNP, showing a significant level in 5 μg/ml of the prednisolone treatment group compared to the SNP treatment group (p<0.05) (Fig. 2B).
Increase of COX-2 was significantly induced by SNP treatment compared to control chondrocytes (p<0.01), and COX-2 expression was decreased by prednisolone treatment in a concentration-dependent manner, showing a significant level in 50 μg/ml of the prednisolone treatment group compared to the SNP treatment group (p< 0.05) (Fig. 2C).
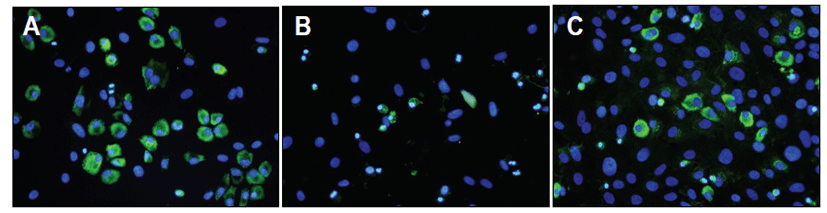
Immunofluorescence staining of type II collagen showed that SNP treatment induced a decrease of type II collagen expression and prednisolone treatment recovered its expression compared with SNP-treated chondrocytes (Fig. 3).
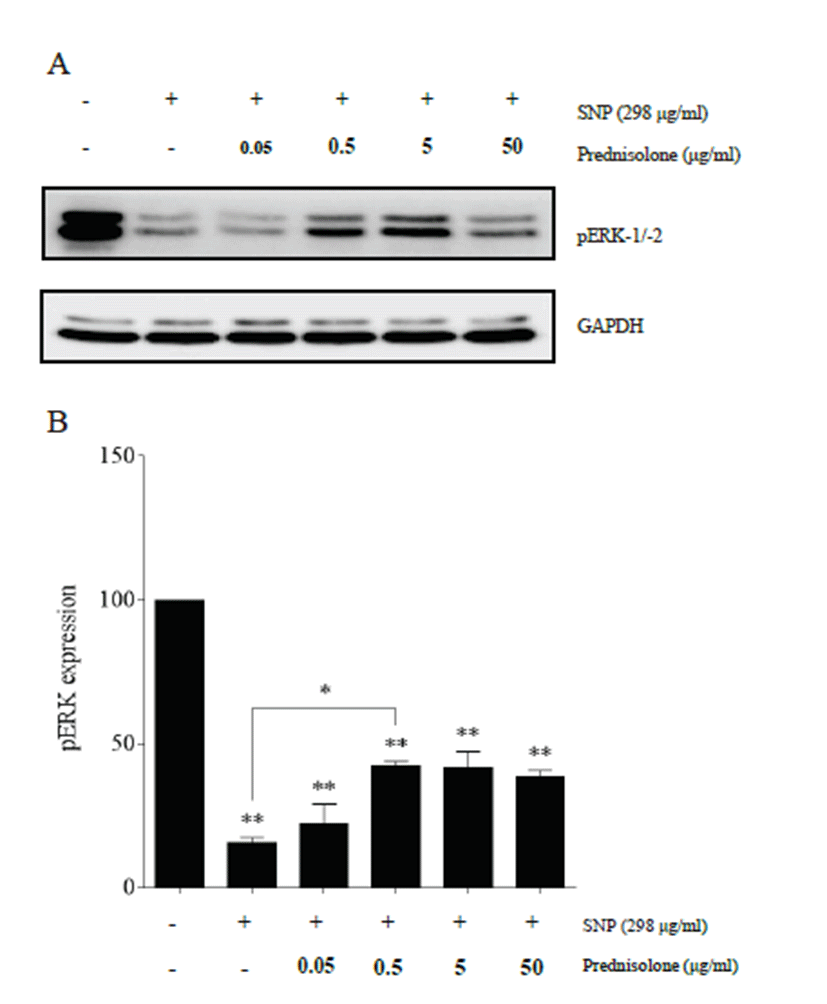
Western blot analysis showed that SNP and prednisolone treatment modulated pERK expression in rabbit articular chondrocytes (Fig. 4A). SNP treatment significantly induced a decrease of pERK expression compared to control chondrocytes (p<0.01), and prednisolone treatment recovered its expression in a concentration-dependent manner, showing a significant level in 0.5 μg/ml of the prednisolone treatment group compared to the SNP treatment group (p<0.05) (Fig. 4B).
Discussion
In this study, SNP treatment induced a decrease of type II collagen compared with control chondrocytes, and prednisolone treatment increased the down-regulated expression of type II collagen induced by SNP in rabbit articular chondrocytes. As type II collagen is one of chondrocyte differentiation markers [1], this result suggests that prednisolone prevented dedifferentiation in rabbit articular chondrocytes treated with SNP. Cartilage extracellular matrix is composed mainly of proteoglycans including the major proteoglycan, aggrecan, and other minor proteoglycans and collagens such as type II collagen and other minor collagens [12]. As they lose their round morphology and change to a spindle shape, collagen II and aggrecan dramatically decrease in contrast to the increase in collagen I [13]. In this study, immunofluorescence staining of type II collagen confirmed the recovery action of prednisolone on SNP-treated chondrocytes. As the preservation of chondrocyte characteristics or prevention of dedifferentiation is one of the important steps for the treatment of arthritis, it seems that prednisolone treatment may have a useful function in cartilage regeneration.
In this study, the SNP treatment induced an increase of COX-2 and the prednisolone treatment increased downregulated COX-2 expression induced by the SNP treatment in chondrocytes. As COX-2 knockout mice showed down-modulation of the development of arthritis [14], reduced COX-2 expression may be associated with inhibition of arthritis progression. So, the prednisolone treatment inhibited the SNP-induced COX-2 expression, showing inhibition of inflammation in chondrocytes. In this study, SNP treatment induced a decrease of pERK expression, and prednisolone treatment increased downregulated their expression induced by SNP treatment. Further studies are warranted to investigate the cellular regulatory mechanism of COX-2 and pERK expression induced by prednisolone treatment.
In this study, the SNP treatment decreased cell viability compared with the control. This was in keeping with NO's mediation in the regulation and survival of the chondrocyte [8]. In this study, the prednisolone treatment decreased SNP-induced cell death in a concentration-dependent manner, but not to a significant level compared to the SNP-treated group. This indicated that prednisolone could not prevent NO-induced cell death directly. Therefore, inhibition of cell death by the prednisolone treatment is considered to be partial or minimal.
As proliferation plays a role in forming characteristic chondrocyte clusters near the surface [1], tracing the position or location of cell proliferation is important in the evaluation of therapeutics. Even though histopathological approach is a valuable and direct method for evaluation of therapeutics [15], it may limit the quantitative and sequential pathogenesis information, especially in the cartilage. Therefore, simultaneous analyses of cartilage parameters, some bioimaging techniques may be useful for simultaneous analyses of cartilage parameters, Bioimaging techniques such as computed tomography [16], magnetic resonance imaging [17], and optical tomography [18] have been used for assessing disease progression. For optic bioimaging, several probes have been used to provide early diagnosis and to get visualization of arthritis progression. These include a bone-specific polymeric probe [19] and a near infrared spectroscopic probe [20]. Furthermore, by enabling the identification of cellular and nuclear features, the resolution provided by optical coherence microscopy makes it possible to distinguish between normal and abnormal lesions [21]. Further studies are warranted to evaluate effects of the prednisolone on arthritis using bioimaging system simultaneously.
Taken the above results together, prednisolone inhibited SNP-induced cell death and dedifferentiation and modulated expression of COX-2 and pERK in rabbit articular chondrocytes.







