Introduction
p63, a member of the p53 gene family, is a transcription factor associated with epidermal morphogenesis and cellular homeostasis by regulating cell proliferation, differentiation and apoptosis [1, 2]. p63 is also considered to be implicated in epidermal carcinogenesis, although its function as a tumor suppressor gene or proto-oncogene is still not fully understood. p63 is also expressed in skin appendages such as fair follicle, sebaceous glands, and sweat glands [3]. Since p63 is usually expressed in putative stem cells, it has been considered as a stem cell marker [4]. However, the functions of p63 in various tissues, in particular hair follicles, have only speculated.
A hair follicle is a sensitive mini-organ with the repetition of cyclic transformation from a rapid growth phase (anagen) to a regression phase (catagen) and quiescent phase (telogen) [5]. The stages of the hair cycle are further classified into sub-stages depending on the morphological, histochemical and immunohistochemical characteristics of hair follicles. The anagen stage is sub-classified into six sub-stages, and the catagen is further divided into eight sub-stages [5]. But, upon light microscopic examination, the classification of the stages of hair follicle is based on typical morphological characteristics, and other supporting criteria such as hair follicle length and location of dermal papillae, although it is difficult to differentiate transition phases from one stage to another subsequent stage. The hair cycle is profoundly regulated by a variety of factors including growth factors, cytokines, hormones, neuropeptides and so on [5]. For normal cycling of hair follicles, stem cells should be maintained as a cellular component of the hair follicle during hair maturation to the telogen stage and should be re-activated for cell proliferation and initiate the anagen stage. Apoptosis is a key event in the transition from the anagen to the catagen stage and subsequently from the catagen to the telogen stages [6, 7]. Thus, the maintenance of hair stem cells and balanced regulation of cell proliferation and apoptosis are critical for the achievement of the normal cycling of hair follicles.
Based on this background, p63 could play an important role in the cycling of hair follicles, in particular, for the maintenance of stem cells and cell proliferation. In the present study, we investigated the expression of p63 in association with cell proliferation in hair follicle cells at any stage of hair cycling in mice, by performing immunohistochemistry for p63 and Ki-67, a marker of cell proliferation [8].
Materials and methods
This animal study was approved by the Institutional Animal Care and Use Committee of Kangwon National University. For the study, seven to eight week-old male C3H/ he mice were purchased from Orient Bio Co., Ltd. (Seongnam, South Korea). After at least 1-week acclimatization, the animals were housed in a temperature-controlled animal facility with a 12-h light-dark cycle throughout the study. During the study, the animals were given standard diet and water ad libitum.
For induction of hair cycling, the back hairs of the mice were depilated by shaving using the razor blades. On day 0, 1 and 7 after shaving, the skin was excised and fixed in 10% buffered formalin. The skin tissues were trimmed and processed for paraffin format, and then sectioned 3 ㎛ in thickness. Hematoxylin and eosin (HE) staining was performed for histological examination, and the sections for immunohistochemistry were selected.
Based on the histological examination, the sections with typical hair cycle stages were selected for immunohistochemistry for p63 and Ki-67. The selected paraffin-embedded skin sections were de-paraffinized in xylene and rehydrated by passage through decreasing concentrations of ethanol. Antigen retrieval was performed by heating the sections in 10 mM sodium citrate buffer (pH 6.0) for 20 minutes using a microwave. Nonspecific binding of antibodies was prevented by incubating sections for 1 hour at room temperature with 1% normal serum albumin in TBS containing Triton X-100 (0.025%). The sections were then respectively incubated with the diluted anti-p63 antibody [4A4] (1:300, mouse monoclonal, Cat.# ab735, abcam Inc., Cambridge, MA, USA) and anti-Ki-67 antibody (1:250, rabbit monoclonal anti-human Ki-67, Cat.#DRM004, Acris Antibodies GmbH, Herford, Germany) overnight at 4°C, and treated with 0.03% hydrogen peroxide in TBS for 20 minutes at room temperature to quench the activity of endogenous peroxidases. For the negative controls, PBS was applied to the tissue sections instead of the primary antibodies. The sections were then incubated with a biotinylated secondary antibody (Vector ABC Elite, Burlingame, CA, US) for 1 hour at room temperature, followed by incubation with an avidinbiotin complex (Vector ABC Elite, Burlingame, CA, US). 3,3′-Diaminobenzidine (Dako, Carpinteria, CA, US) was used as chromogen, and the sections were counterstained with Mayer’s hematoxylin (Dako, Carpinteria, CA, US).
Results
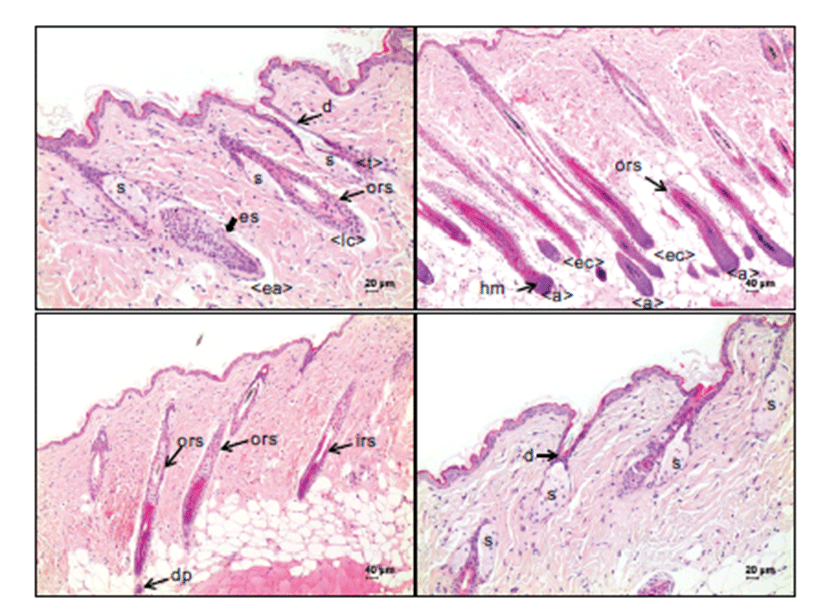
The classification of hair follicle stages was determined by modification of the criteria provided by Müller-Röver’s study [5]. Although it was not easy to determine the stage when the hair follicles were in a transition phase between stages, the anagen stage of a hair follicle is morphologically characterized by the development of an enlarged, onionshaped hair bulb composed of matrix cells, melanocytes and keratinocytes (Fig. 1). The transition from quiescent phase (telogen) to growth phase (anagen) is initiated by the thickening and prolongation of the epithelial strand, which is the column of keratinocytes between the germ capsule and the compact dermal papilla (Fig. 1A). At this early anagen stage, the proximal end of the hair follicle with a developing hair bulb and dermal papilla is located around the border between the dermis and the subcutis. In the middle and late anagen stages, the hair bulb, which is growing larger to reach its maximum size, resides in the subcutis (Fig. 1B). The transition from the anagen to the regression phase (catagen) is determined by a narrowing hair bulb and dermal papillae (Fig. 1B & 1C). In the catagen stage, the dermal papillae become ball-shaped, not completely enclosed by narrow hair bulbs, and its location ranges from the border between the dermis and subcutis to the dermis. In the quiescent phase (telogen stage), no internal root sheath is observed, the ballshaped dermal papillae are attached to the germ cell cap, and it is fully located in the dermis and reaches the sebaceous gland level (Fig. 1D).
In anagen stages, many hair matrix cells in the hair bulbs, surrounding the dermal papillae, and few outer root sheaths along the hair follicles indicated strong nuclear immunoreactivity to p63 (Fig. 2B). The melanocytes in the hair bulbs were heterogeneously immunoreactive for p63. The strand cells of keratinocytes in the early phase of the anagen stage, which were commonly noted on Day 1 after depilation, were strongly positive for p63 (Fig. 2A). However, inner root sheath cells and mesenchymal cells in the dermal papillae were negative for p63 in the hair follicles during the anagen stage.
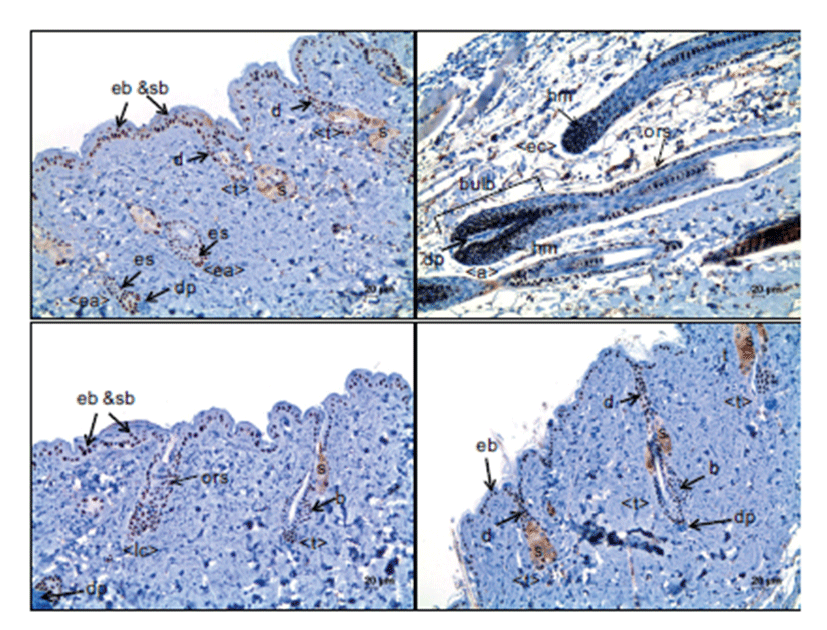
The expression pattern of p63 in the early catagen stage was similar to that for anagen stage follicles; matrix cells in the hair bulb and outer root sheath cells were positive, but inner root sheath cells were negative for p63 (Fig. 2B). In the late catagen stage, p63 was expressed in the outer root sheath cells, seboblasts and duct cells (Fig. 2C). However, inner root sheath cells and the mesenchymal cells of dermal papillae were consistently negative for p63.
In the telogen stage, the hair germ keratinocytes forming the small cap that is closely attached to small, compact, ballshaped dermal papillae were strongly immunoreactive for p63. Outer root sheath cells were also positive for p63, like those in hair follicles in the anagen and catagen stages. In the sebaceous glands that are attached to the hair follicles at the top of the dermis, seboblasts located at the periphery of the glands and duct cells continuous to the basal cell layer of skin epidermis were also positive for p63 (Fig. 2).
In the anagen stage of hair follicles with well-developed hair bulbs, hair matrix cells surrounding the dermal papillae were positive for Ki-67 (Fig. 3B), indicating that they are in active cell proliferation. Only a few outer root sheath cells, approximately less than 5%, were Ki-67-positive in the suprabulbar, isthmus and infundibulum regions of hair follicles. Inner root sheath cells and mesenchymal cells of dermal papillae were negative. In the early anagen stage, epithelial strand cells indicated Ki-67 immunoreactivity with a relatively high labeling index, up to around 20% (Fig 3A).
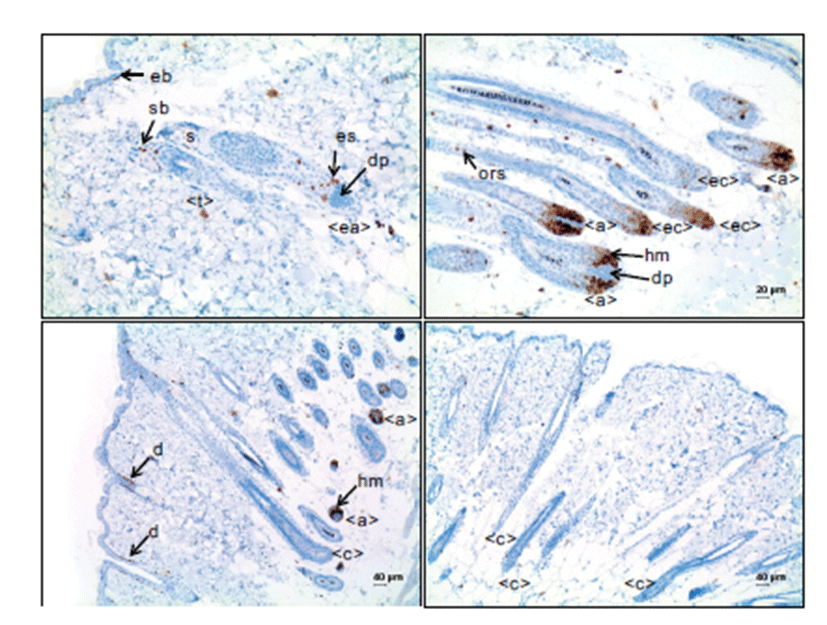
In the catagen stage of hair follicles, the hair matrix cells in the narrowed hair bulbs were positive for Ki-67, but the positive cells notably reduced in number compared to those in anagen stage hair follicles with well-developed hair bulbs, associated with the decreased number of matrix cells (Fig. 3B). Outer root sheath cells were rarely positive for Ki-67, in a range of ~1%; the number of Ki-67-positive cells was less than that in those cells of the hair follicles in the anagen stage. In the middle and late catagen stage, out root sheath cells were mostly negative for Ki-67 (Fig. 3C and 3D).
In the telogen stage of hair follicles, a small number of outer root sheath cells were positive for Ki-67. Seboblast cells at the periphery of the sebaceous glands were positive for Ki-67 (Fig. 3A). The duct cells continuous to the basal cells of the epidermis also indicated Ki-67 immunoreactivity, although the labeling index was variable.
Discussion
Expression of p63 is required for normal epidermal morphogenesis by participating in the commitment of singlelayered surface ectoderm cells to stratification [9]. In the mature epidermis, p63 is also known to play significant roles in the maintenance of basal stem cells and regulation of cell proliferation. The hair follicle is a skin appendage organ, developed from epidermal stratification and downward growth [10]. Once hair follicles are developed, they undergo cycles of growth, regression and quiescent phase for life. In the normal cycling of hair follicles, the counter-balanced regulation of cell proliferation, differentiation and apoptosis are essential [5], although the underlying mechanism is still being speculated. In the present study, the expression pattern of p63 in hair follicle cells at different cycle stages was investigated, and it was speculated that p63 expression was related to cell proliferation activity.
In the present study, immunohistochemistry for p63 indicated a clear nuclear staining of the target cells. In hair follicles in the telogen stage, the cells in the bulge which is at the point of insertion of the arrector pili muscle and the distal outer root sheath cells which are continuous to the epidermal basal cells were immunoreactive for p63. In hair follicles in the early anagen stage, the cells of the epithelial strand, which was formed by the proliferation of hair stem cells in the bulge, were positive for p63. In hair follicles with well-developed hair bulbs, p63 was intensely expressed in the matrix cells surrounding the dermal papillae and outer root sheath cells. However, inner root sheath and dermal papilla cells were negative for p63. These findings were in accordance with the previous study [3], although they did not address the expression of p63 in the detailed parts of hair follicles at different hair cycles. In the present study, the expression pattern of p63 was associated with the movement of hair follicle stem cells. Hair follicle stem cells are mainly located in the bulge in the telogen stage and, as the hair follicles go into the anagen stage, they proliferate, expand and form the epithelial strands of hair follicles and continuously grow down to form the hair bulbs [11]. In this study, p63 was strongly expressed in the interfollicular epidermal basal cells and seboblasts, supporting the idea that p63 is a stem cell marker [12]. In the present study, the positive expression of p63 was dependent on cell components rather than hair follicle stage. For instance, outer root sheath cells were immunoreactive for p63 in all stages of the hair cycle. On the contrary, the expression of Ki-67 was dependent on the hair cycle. In hair follicles in the telogen stage, no positive cells were observed, except for the seboblasts and the duct cells continuous to the epidermal basal cells. Also, the hair follicle cells in the catagen stage were negative or very rare for Ki-67. Cell proliferation in the hair follicles mainly occurred during the anagen stage. The relatively high Ki-67-labeled cells were evident in the epithelial strand in the early anagen stage. The Ki-67-positive cells markedly increased in the hair follicles in the anagen stage with welldeveloped hair bulbs; most of the matrix cells in the hair bulbs were Ki-67-positive, indicating their high proliferation activity. In hair follicles in the anagen stage with welldeveloped hair bulbs, some outer root sheath cells in the suprabulbar and isthmus regions were Ki-67-positive, compared to no positive outer root sheath cells in hair follicles in the catagen stage. Concerning the relation between p63 expression and Ki-67, Ki-67 expression coincided with p63 expression in the cell components of hair follicles, although p63-expressed cells were not always positive for Ki-67. Our results suggested that keratinocytes that have the ability to proliferate when necessary are among the p63-positive cells in the hair follicles.
There are two main isoforms in p63, TAp63 and ΔNp63 depending on the presence of an N-terminal transactivation domain; the functions of p63 are achieved by their counterbalancing in expression [13]. ΔNp63 is predominantly expressed in skin basal cells, and is considered to play an essential role in maintaining the proliferative capacity of putative stem cell populations [14]. The p63 antibody we used in this study was designed to detect both types of p63, but the expressions seemed to be ΔNp63, as Tsujita-Kyutoku et al. (2003) and Romano et al. (2010) previously indicated similar results to ours [3, 15]. According to the in vitro study using basal keratinocytes, it was shown that ΔNp63 induces cell proliferation and prevents the premature initiation of cell differentiation [16], thereafter clearly evidenced in the ΔNp63 transgenic mice [15]. The correlation of p63 expression with cell proliferation capacity was also postulated in other studies [4, 17, 18]. In the studies, it was shown that p63 was required for cell proliferation, and its loss induced cell cycle arrest [17, 18]. The effect of p63 in promoting cell proliferation and the maintenance of stem cell properties by p63 was considered to be associated with its function to inhibit p53, p21 and Notch [19, 20]. This hair follicle development has been shown to be achieved by implication with a variety of molecules including p63 [21], but the underlying mechanisms of hair follicle cycling in mature skin remain veiled in many areas. Orchestrating counterbalanced expressions of various genes will be required for the normal cycling of hair follicles which includes stem cell regulation, proliferation and apoptosis of transient amplifying cells, differentiation into hair follicle cell components, and other delicate molecular events. Further studies are needed to elucidate the molecular mechanisms of hair follicle cycling.
In summary, in this study, it was found that p63 was expressed in the bulge stem cells, epithelial strand cells, matrix cells and outer root sheath cells of hair follicles at any stage of the cycle, which was associated with the movement of hair progenitor cells for regeneration. Ki-67-positive cells were evident among the p63-expressing cell components. Our results strongly suggest that p63 plays an important role in stem cell regulation, at least associated with cell proliferation, for the regeneration of hair follicles.







