Original Article
Reactive oxygen species (ROS) induces distinct gene expression in human fibroblast like synoviocytes (HFLS)
Kyung Min Kil2
,†,
So-Hee Jung1
,†,
In-Sook Jeon1,
Byung-Ki Cho2,
Ji Kang Park2,
Kyung-Jin Park2,
Yong-Min Kim2
,*,
Joong-Kook Choi1
,*

Author Information & Copyright ▼
1Department of Biochemistry, College of Medicine, Chungbuk National University, Cheongju 28644, Korea
2Department of Orthopaedic Surgery, College of Medicine, Chungbuk National University, Cheongju 28644, Korea
† These authors contributed equally to this work.
*Corresponding author: Joong-Kook Choi Department of Biochemistry, College of Medicine, Chungbuk National University, Cheongju 28644, Korea +82-43-261-2844, +82-43-273-9710,
jkchoi@chungbuk.ac.kr Yong-Min Kim Department of Orthopaedic Surgery, College of Medicine, Chungbuk National University, Cheongju 28644, Korea +82-43-269-6359,
ymkim@chungbuk.ac.kr
© Research Institute of Veterinary Medicine, Chungbuk National University. All rights reserved.
Received: Sep 29, 2017; Revised: Dec 14, 2017; Accepted: Dec 16, 2017
Abstract
Adhesive capsulitis of the shoulder is a common cause of pain that occurs during shoulder movement, thereby restricting shoulder rotation in clinical practice. Although most patients respond to pain relief treatment (NSAID or corticosteroids) by improving their range of motion, it remains poorly understood without any definitive treatment algorithm. In addition to immune cells, synoviocytes, chondrocytes and osteoblasts in the joint are known to produce pro-inflammatory mediators such as reactive oxygen species (ROS), inflammatory cytokines and lipid mediators, presumably contributing to the pathogenesis of osteoarthritis (OA) and adhesive capsulitis. Although inflammation and also fibrosis are proposed to be the basic pathological changes of a frozen shoulder, there is a lack of information regarding the downstream targets of the pro-inflammatory ROS signaling pathway in the synoviocytes and also how these ROS targets are modulated at the transcription level by a corticosteroid - dexamethasone. In this study, we used human fibroblast like synoviocytes (HFLS) to characterize the signaling targets of ROS by employing a human DNA microarray tool and studied the role of dexamethasone in this process. Our data suggest that several genes such as FOS, FOSB and NFkBIZ, which are known to be involved in pro- or anti- inflammation response, are modulated at the transcription level by ROS and dexamethasone.
Keywords: synoviocytes; reactive oxygen species; dexamethasone; adhesive capsulitis; DNA microarray
Introduction
Primary frozen shoulder (FS) is known to be a painful contracture of the glenohumeral joint that arises spontaneously without an obvious preceding event [1].
Although the cause of frozen shoulder still remains unclear, molecular biological studies demonstrated that inflammation and fibrosis are the basic pathological changes of FS [2]. This seems to be related to an increased amount of collagen, fibrotic growth factors such as TGF-beta, and inflammatory cytokines like tumor necrosis factor-alpha and interleukins. Additionally, B and T lymphocytes and macrophages appear to be associated with FS [1].
Multi-factorial etiology is reported in osteoarthritis to include oxidative stress. The overproduction of ROS may regulate an intracellular signaling processes, chondrocyte senescence and apoptosis, extracellular matrix (ECM) synthesis and degradation along with synovial inflammation and ultimately fibrosis [3, 4].
Investigation of the rotator interval capsule and coracohumeral ligament obtained from FS patients disclosed active fibroblastic proliferation accompanied by some transformation to myofibroblasts. However, at least with inflammation and synovial involvement in a resemblance to Dupuytren’s disease [5,6], fibroblast-like synoviocytes (FLS) are a special cell type located inside joints in the synovium and are presumably involved in chronic inflammatory diseases including rheumatoid arthritis [7].
Current medication for FS patients includes either nonsteroidal anti-inflammation drugs (NSAID) or corticosteroids such as dexamethasone (Dexa). Injections of corticosteroids can provide rapid pain relief in the short term (particularly in the first 6 weeks), but long-term outcomes are known to be close to other treatments including placebos [8].
Nevertheless, there is a dearth of reports about ROS signaling and the role of anti-inflammation drugs in this process in the synoviocytes. We aimed to investigate this relationship using DNA microarray and RT-PCR methods in hydrogen peroxide-treated and/or dexamethasonetreated synoviocytes.
Materials and Methods
Cell culture and reagents
Human fibroblast-like synoviocytes (HFLS), which are derived from normal healthy human synovial tissue, were purchased from Cell Applications Inc. (Sandiego, CA). They were cultured in HFLS media (Cell Applications Inc.) at 37 °C in a humidified atmosphere containing 5% CO2. Dexa was purchased from Sigma Chemical Co. (St Louis, MO) and a medical grade H2O2 was purchased in a local pharmacy.Fig. 1
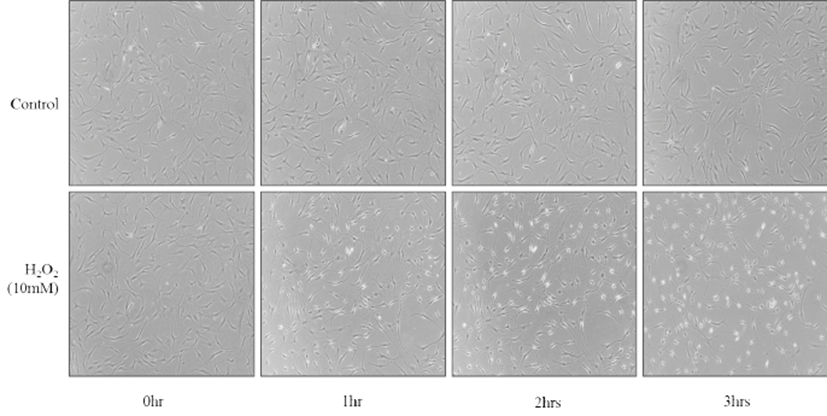
Fig. 1.
Morphological change of HFLS after treatment with H2O2 over the 3 hour course. HFLS cells were plated in 6 well plate at a cell density of 5x104 and after overnight, they were treated with 10mM H2O2 for 0, 1, 2, and 3 hrs before imaging with Olympus IX71 microscope
Download Original Figure
DNA microarray
Illumina Human HT-12 v4 Expression BeadChip (Illumina, Inc., San Diego, CA), which includes about 47,000 genes, was used to analyze the gene expression profile of the HFLS cells. After stimulation with H2O2 for 2 hrs, total RNAs were extracted with RNA Blood Mini Kit (QIAGEN, Hilden, Germany), and equal amount of total RNAs was analyzed for differentially expressed genes by the gene chip and normalized by MAS5 normalization (Macrogen, Seoul, Korea).Table 1
Table 1.
List of genes that are differentially expressed in the DNA microarray
| NCBI No. |
Gene (Homo sapiens) |
Gene symbol |
Fold Change |
| NM_005252.2 |
Fos proto-oncogene, AP-1 transcription factor subunit |
FOS |
15.6 |
| NM_006732.1 |
FBJ murine osteosarcoma viral oncogene homolog B |
FOSB |
9.4 |
| NM_015675.2 |
Growth arrest and DNA-damage-inducible, beta |
GADD45B |
2.5 |
| NM_002228.3 |
Jun proto-oncogene, AP-1 transcription factor subunit |
JUN |
2.2 |
| NM_002648.2 |
Pim-1 oncogene |
PIM1 |
1.8 |
| NM_006622.2 |
Polo-like kinase 2 |
PLK2 |
1.7 |
| NM_000399.2 |
Early growth response 2, transcript variant 1 |
EGR |
1.6 |
| NM_001005474.1 |
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta, transcript variant 2 |
NFKBIZ |
1.6 |
Download Excel Table
Reversre Transcriptase (RT) PCR
HFLS cells were seeded in 100 mm dish at the density of 5x106/dish and after overnight, treated with H2O2 (10 mM final concentration) and/or Dexa (1 μM) for 2 hrs. Total RNAs were extracted with RNA Blood Mini Kit (QIAGEN) before cDNA synthesis with AccuPower rocket script RT/PCR premix (Bioneer, Daejeon, Korea) and10 ng of total RNA and 10 μM of 5’ and 3’ primers (each) in a total volume of 20 μL. PCR reaction was proceeded a follow; cDNA synthesis for 42°C/40 min, predenaturation for 95°C/5 min, followed by 35 cycles of denaturation for 95°C/30 sec, annealing for 54°C/30 sec, extension for 72°C/30 sec. 10 ul of PCR reaction was run on 1.5% agarose gels for electrophoresis and stained with ethidium bromide staining for image analysis.
Results
H2O2 induces morphological change of HFLS cells over 3 hrs
In order to investigate whether H2O2 may induce any cell shape changes or apoptotic cell death over time, the HFLS cells were treated with 10 mM H2O2 for various lengths of time: from 1 to 3 hrs. As reported previously, 10 mM H2O2 induced a morphological change as early as 1 hr after treatment and later cell death. Since a high concentration of H2O2 is known to induce cell death [9], we chose this concentration of H2O2 in the subsequent DNA microarray experiment to identify cell stress response against pro-inflammatory ROS.
Differential expression of H2O2 responsive genes
After HFLS cells were treated with normal growth media (control) or 10 mM H2O2, total RNAs were extracted and analyzed with Illumina HumanHT-12 v4 DNA array chip containing about 47,000 genes. From the DNA array data, we found that 106 and 32 genes were up-regulated by more than 1.5 folds and 2 folds respectively, whereas 135 and 20 genes were down-regulated in H2O2 treated cells compared to those of control cells (result not shown). From the up-regulated genes, we chose 12 genes which include FOS, FOSB, GADD45B, JUN, PIM1, PLK2, NFKBIZ, OSR2, EGR2, LOC338758, GDF15 and LOC387763, whose functions are reported or unknown for further analysis by RT-PCR. Out of 12 genes, we could not identify a PCR amplified band with PCR primers specific for LOC338758, GDF15 or LOC387763 cDNAs (result not shown).
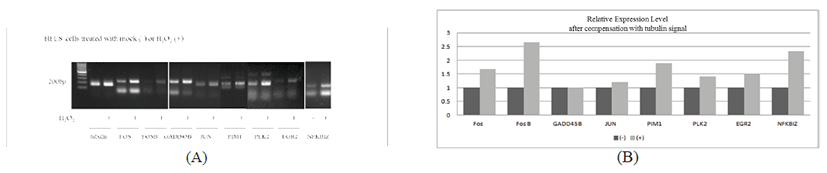
Since we noticed that the tubulin controls were differentially expressed by 50% between mock (-) control and H2O2 treated samples, we chose the sum of 28S- and 18S-rRNA signal as an internal loading control (supplementary result). After compensation of the two rRNA signals between the two samples, the expression levels of 9 genes were adjusted accordingly, as shown in Fig. 2B.
Fig. 2.
Differential expression of H2O2 responsive genes in HFLS Cells. HFLS cells were grown in normal growth media before addition of either plain media or 10 mM of H2O2 for 2 hrs. Thereafter, total RNAs were extracted for RT-PCR using specific primers for the genes screened from DNA microarray analysis. Tubulin was included as an internal control of equal loading of total RNA samples. (A) The signal intensity, in each lane on the agarose gel containing the PCR product of the expected size, was quantitated by Image Lab 5.0 and presented as graph (B).
Download Original Figure
From these analyses, FOS, FOSB, NFKBIZ and OSR2 appear to be transcriptionally up-regulated by more than 50% in the H2O2 samples, whereas JUN, PIM, PLK2 and EGR2 exhibited a modest up- or down-regulation in the H2O2 samples.Table 2
Table 2.
List of primers for RT-PCR analysis
| Gene symbol |
Sequences of primers (5’ - 3’) |
Location |
Size of PCR products(bp) |
| FOS |
CCAACCTGCTGAAGGAGAAG |
556 |
233 |
| GCTGCTGATGCTCTTGACAG |
788 |
| FOSB |
AGGAAGAGGAGAAGCGAAGG |
458 |
225 |
| CTTCGTAGGGGATCTTGCAG |
682 |
| GADD45B |
GTGTACGAGTCGGCCAAGTT |
118 |
249 |
| GACCAGGAGACAATGCAGGT |
366 |
| JUN |
GAGCGGACCTTATGGCTACA |
66 |
192 |
| CCGTTGCTGGACTGGATTAT |
257 |
| PIM1 |
CAGAGTGGATCCGCTACCAT |
629 |
226 |
| TGGATTTCTTCGAAGGTTGG |
854 |
| PLK2 |
CAGCAGATTGGGGATGCTAT |
1315 |
203 |
| TTGGTGACCCACTGAAATGA |
1517 |
| EGR2 |
ATTCTGAGGCCTCGCAAGTA |
958 |
259 |
| TTCGGCCACAGTAGTCACAG |
1436 |
| NFKBIZ |
GCCTAATTCAAATGGGAGCA |
1490 |
201 |
| TCAACCGATACTGCAAGCTG |
1690 |
Download Excel Table
Modulation of H2O2 responsive gene expression by Dexa
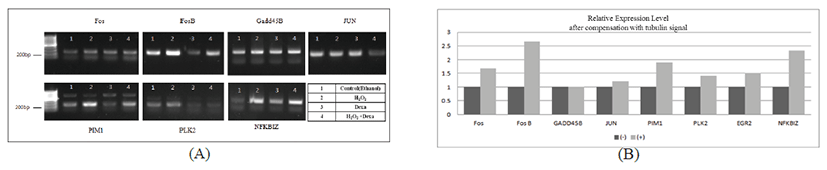
Based on the data in the above, we sought to study whether a Dexa, one of the most commonly prescribed corticosteroids for pain and osteoarthritis, can modulate the expression of the ROS responsive genes. For this purpose, HFLS cells were treated with ethanol (mock), H2O2, Dexa or H2O2 + Dexa for 2 hrs and subjected to RT-PCR analysis. As shown in Fig. 3A, Dexa appears to down-regulate the expression of FOSB, PIM1 and PLK2, while it increases the expression level of NFKBIZ irrespective of H2O2 . Meanwhile, the expression of JUN seems to be inhibited only when HFLS cells were treated with both H2O2 and Dexa.
Fig. 3.
Effect of Dexa on the H2O2 responsive gene expression. HFLS cells were grown in normal growth media before they are simulated with either plain media, 10 mM H2O2, and/or 1 uM Dexa for 2 hrs . Subsequently, total RNAs were extracted and used for RT-PCR using gene specific primers before agarose gel run (A). Individual signal intensity in (A) was quantified by Bio Lab 5.0, and the signal in each control lane was used as an reference for equal loading before presenting as graphs in B.
Download Original Figure
Discussion
Main pathology of primary adhesive capsulitis has been reported to be an inflammatory contracture of the shoulder joint capsule resulting from synovitis and subsequent fibrosis [1]. Although several biomarkers including TGF-β, PDGF, TNF-α, IL-1β and COL3A1 are demonstrated to be associated with the synovial hyperplasia and capsular fibrosis of the affected tissues, their precise role in the pathological process is not clear [10,11].
Recent studies have identified both oxidative stress and ROS to be significantly associated with osteoarthritis (OA) progression [12, 13]. Therefore, unraveling ROS signaling is proposed to offer a valuable perspective for exploration of potential therapeutic strategies in the treatment of OA [3]. However, the role of ROS signaling in adhesive capsulitis remains unknown and in this study, we attempted to characterize the role of oxidative stress in a human synoviocyte cell line (HFLS) using H2O2 .
HFLS cells showed a morphological change at 10 mM of H2O2 starting from 1 to 3 hrs and thus, we choose this condition (10 mM and 2 hr) in the subsequent DNA microarray. To chart an expression profile of whole human genome, we used an Illumina HumanHT-12 v4 Expression BeadChip covering 25,000 annotated genes. Out of 106 genes, which exhibited an increased expression in H2O2 -treated HFLS by more than 1.5 folds, we chose 12 genes to confirm the expression by using the reverse transcriptase PCR method and could get the PCR products from 9 genes. The 3 exceptions were (GDF15 (NM_004864.1), LOC338758 (XM_931359.2), and LOC387763 (XM_941665.2)). This RT-PCR analysis revealed that ROS stress could elicit a differential expression of novel genes such as FOS, FOSB, PIM1, NFKBIZ, PLK2 and OSR2 by about 1.5 folds. On the other hand, GADD45B, JUNB and PLK2 turned out to be unresponsive to H2O2 stimulation at the transcription level (Fig. 2A and 2B).
FOS and FOSB constitute AP1 transcriptional regulator involved in inflammatory bone and skin [14, 15] and rapidly is up-regulated by inflammatory cytokines such as TNF and IL-6 [16]. In contrast, Pim1 kinase is reported to have both pro- and anti-inflammatory activities depending on cellular backgrounds and the nature of stimuli [17,18], NFKBIZ is a nuclear inhibitor of NFkB that is induced by lipopolysaccharide or interleukin-1, and its role in the suppression of atopic dermatitis was recently demonstrated [19, 20]. PLK2 appears to be involved in high glucose induced inflammation in podocytes [21], while OSR2, a zinc finger transcription factor involved in mouse development and joint formation, does not have any apparent role in inflammation [22,23]
Corticosteroids are often recommended for adhesive capsulitis and osteoarthritis to control inflammation, pain and to inhibit tissue destruction [24]. However, corticosteroids in adhesive capsulitis are reported to confer a protective effect only in the short-term - less than 12 weeks [25, 26]. We studied whether a corticosteroid- Dexa could modulate the expression of the ROS target genes in the above. Compared to FOS, the expression of pro-inflammatory FOSB, PIM1 and PLK2 appeared to be inhibited by 1 uM of Dexa, whereas the expression of GADD45 and JUN was inhibited only when both ROS stress and Dexa were present. NFKBIZ expression increased by more than 2 folds in the presence of Dexa and it will be interesting to study whether this NFkB inhibitor plays a critical role in anti-inflammatory activity of Dexa in adhesive capsulitis.
Our data in this study present novel biomarkers, which are known to be involved in pro- or anti-inflammatory cellular signaling, and future study is necessary to identify their respective or collective role in adhesive capsulitis.
Supporting information
200 ng of total RNAs was electrophoresed in agarose gel and the relative signal intensity of tubulin in each sample was quantified by Image Lab 5.0 (Biorad, USA). After compensation of H2O2, Dexa and H2O2/Dexa signals to that of control, individual gene expression was presented in Fig. 2 and Fig. 3..
Acknowledgements
This work was supported by the intramural research grant of Chungbuk National University in 2015 and Next Generation BioGreen21 project (Project No.PJ01104401 and PJ01110901) of Rural Development Administration, Republic of Korea to Joong-Kook Choi.
References
Tamai K., Akutsu M., Yano Y. Primary frozen shoulder: brief review of pathology and imaging abnormalities.. J. Orthop. Sci. 2014; 19:1-5.

Cui J., Lu W., He Y., Jiang L., Li K., Zhu W., Wang D. Molecular biology of frozen shoulder-induced limitation of shoulder joint movements.. J. Res. Med. Sci. 2017; 22:61.

Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis.. Biochim Biophys Acta. 1862; 1862:576-591.

Richter K., Konzack A., Pihlajaniemi T., Heljasvaara R., Kietzmann T. Redox-fibrosis: Impact of TGFbeta1 on ROS generators, mediators and functional consequences.. Redox Biol. 2015; 6:344-352.

Bunker T.D., Esler C.N. Frozen shoulder and lipids.. J. Bone Joint Surg. Br. 1995; 77:684-686.

Bunker T.D., Reilly J., Baird K.S., Hamblen D.L. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder.. J. Bone Joint Surg. Br. 2000; 82:768-773.

Chang S.K., Gu Z., Brenner M.B. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11.. Immunol. Rev. 2010; 233:256-266.

Song A., Higgins L.D., Newman J., Jain N.B. Glenohumeral corticosteroid injections in adhesive capsulitis: a systematic search and review.. PM R. 2014; 6:1143-1156.

Teramoto S., Matsuse T., Matsui H., Ohga E., Ishii T., Nagase T., Ouchi Y. Recombinant E1-deleted adenoviral vectors induce apoptosis in a rat airway epithelial mucous goblet cell line.. Jpn. J. Pharmacol. 1999; 81:73-78.

Rodeo S.A., Hannafin J.A., Tom J., Warren R.F., Wickiewicz T.L. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder.. J. Orthop. Res. 1997; 15:427-436.

Nago M., Mitsui Y., Gotoh M., Nakama K., Shirachi I., Higuchi F., Nagata K. Hyaluronan modulates cell proliferation and mRNA expression of adhesion-related procollagens and cytokines in glenohumeral synovial/ capsular fibroblasts in adhesive capsulitis.. J. Orthop. Res. 2010; 28:726-731.

Henrotin Y., Kurz B., Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes?. Osteoarthritis Cartilage. 2005; 13:643-654.

Li P., Zhang J.F., Li L., Liu Y.H., Shi Y., Wang L.W., Gao J.Q., Liu C. The impact of a family history of type 2 diabetes on insulin secretion and insulin sensitivity in individuals with varying glucose tolerance.. Am. J. Med. Sci. 2013; 345:22-27.

Wagner E.F., Eferl R. Fos/AP-1 proteins in bone and the immune system.. Immunol. Rev. 2005; 208:126-140.

Zenz R., Eferl R., Scheinecker C., Redlich K., Smolen J., Schonthaler H.B., Kenner L., Tschachler E., Wagner E.F. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease.. Arthritis Res. Ther. 2008; 10:201.

McKay S., Bromhaar M.M., de Jongste J.C., Hoogsteden H.C., Saxena P.R., Sharma H.S. Pro-inflammatory cytokines induce c-fos expression followed by IL-6 release in human airway smooth muscle cells.. Mediators Inflamm. 2001; 10:135-142.

Narlik-Grassow M., Blanco-Aparicio C., Cecilia Y., Perez M., Munoz-Galvan S., Canamero M., Renner O., Carnero A. Conditional transgenic expression of PIM1 kinase in prostate induces inflammation-dependent neoplasia.. PLoS One. 2013; 8:e60277.

de Vries M., Heijink I.H., Gras R., den Boef L.E., Reinders-Luinge M., Pouwels S.D., Hylkema M.N., van der Toorn M., Brouwer U., van Oosterhout A.J., Nawijn M.C. Pim1 kinase protects airway epithelial cells from cigarette smoke-induced damage and airway inflammation.. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014; 307:L240-L251.

Ishiguro-Oonuma T., Ochiai K., Hashizume K., Iwanaga T., Morimatsu M. Nfkbiz regulates the proliferation and differentiation of keratinocytes.. Jpn. J. Vet. Res. 2015; 63:107-114.

Ishiguro-Oonuma T., Ochiai K., Hashizume K., Morimatsu M. The role of IFN-gamma in regulating Nfkbiz expression in epidermal keratinocytes.. Biomed. Res. 2015; 36:103-107.

Zou H.H., Yang P.P., Huang T.L., Zheng X.X., Xu G.S. PLK2 Plays an Essential Role in High D-Glucose-Induced Apoptosis, ROS Generation and Inflammation in Podocytes.. Sci. Rep. 2017; 7:4261.

Gao Y., Lan Y., Ovitt C.E., Jiang R. Functional equivalence of the zinc finger transcription factors Osr1 and Osr2 in mouse development.. Dev. Biol. 2009; 328:200-209.

Gao Y., Lan Y., Liu H., Jiang R. The zinc finger transcription factors Osr1 and Osr2 control synovial joint formation.. Dev. Biol. 2011; 352:83-91.

Huebner K.D., Shrive N.G., Frank C.B. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis.. J. Orthop. Res. 2014; 32:566-572.

Song D.H., Chung M.E., Han Z.A., Kim S.Y., Park H.K., Seo Y.J. Anatomic localization of motor points of wrist flexors.. Am. J. Phys. Med. Rehabil. 2014; 93:282-286.

Koh K.H. Corticosteroid injection for adhesive capsulitis in primary care: a systematic review of randomized clinical trials.. Singapore Med. J. 2016; 57:646-657.