Introduction
The pathological causes of benign prostatic hyperplasia (BPH) include inflammatory and cellular proliferative factors. Such signals may lead to hyperplasia of the stromal and glandular tissues in the prostate gland [1-3]. The prostate is located around the male urethra and beneath the urinary bladder, therefore enlargement of the prostate may cause obstructive symptoms in urinations, termed lower urinary tract symptoms (LUTS), which are including frequency, urgency, nocturia, voiding difficulty, and dribbling [4]. However, the prostate is a male specific organ, and its growth is affected by androgenic hormones, such as testosterone and dihydrotestosterone [5-7], and therefore, BPH and LUTS are common diseases that occur after middle-age [4].
Androgen-induced increased signal may include the c- Fos [7-9]. Androgen may not directly induce c-Fos [7], however, androgen receptor may regulate epidermal growth factor (EGF) or insulin like growth factor 1 (IGFI) [10-12], which is closely related to c-Fos signal [13, 14]. The c-Fos may act as a regulator of cell proliferation, differentiation, and transformation [15-17].
Moreover, c-Fos play a role in controlling neuronal activity and survival. Interestingly, it has been suggested that neurological factors cause LUTS [18]. And micturition is under the influence of diverse neurological circuits, because brain, spinal cord, and peripheral nervous system and their neurotransmitters are involved in development of urinatary control [19]. There are evidences suggesting that c-fos is related to the control of micturition [20, 21]. A previous study reported that c-Fos expression is related to bladder reflex micturition [22].
Urinary center of central nervous system includes pontine micturition center (PMC), periaqueductal gray (PAG), and medial preopticnucleus (MPA) [19, 23, 24]. The c-Fos expression has been used as a marker of neuronal activity [25], and c-Fos expression changes in stress urinary incontinence has been reported [26]. These previ ous studies suggest that LUTS may affect the expression of c-Fos [26].
A previous study showed that the Panax ginseng C.A. Mayer (P. ginseng) has a protective effect on enlargement of the prostate in testosterone induced BPH rat model [27]. However, PMC, vlPAG, and MPA regions in brain were not investigated. Therefore, this study investigated the effects of P. ginseng on c-Fos expression in the brain in testosterone induced BPH rat model.
Materials and Methods
P. ginseng is a four year-old Korean ginseng. It was collected from the Department of Medicinal Crop Research (Eumsung-gun, Chungbuk, Korea) in September 2010. To obtain the water extract of ginseng, 100 g of ginseng root was added to 600 mL of distilled water, and the mixture was extracted by heating at 95°C. It was then filtered through a muslin cloth and lyophilized. The resulting powder (yield 32 g) was dissolved in distilled water and sterilized by passing through a 0.22 μM filter sequentially [27, 28].
In the present study, seven-week-old Wistar rats (Central Lab Animal Inc, Korea) were used with an average weight of 250 ± 10 g. The animal room was maintained at 22 ± 2°C and relative humidity of 40~70%. Indoor lighting consists of 12 periods of light and dark cycles. All experiments were carried out in accordance with procedures approved by the Animal Care Committee of the Animal Center at Kyung Hee University and in accordance with guidelines of the Korean National Health Institute of Health Animal Facility.
BPH was induced by subcutaneous injection of testosterone (20 mg/kg) for 4 weeks. Following sentinel resection, rats were divided into four groups (n = 6): (A) control group; (B) BPH derived group subcutaneously injected with testosterone; (C) P. ginseng group treated with 200 mg/kg. (D) 1 mg/kg of the finasteride was orally administered (Sigma-Aldrich, St Louis, MO, USA) as a positive anti-BPH drug. All materials were administered to the animals once a day for 4 weeks, and body weights were measured weekly. After 4 weeks, all animals were fasted overnight. Animals were sacrificed, and fresh prostate was stored in formaldehyde solution for optical microscopy. The remaining prostate was stored at -70°C for later analysis.
Immunostaining was performed with 35 μM sections of brain tissues. Peroxidase activity quenching was performed with 3% H2O2 in PBS for 10 min. The sections were then washed with water and pre-blocked with normal goat or rabbit serum for at least 1 hour. In the primary antibody reaction step, slides were diluted 1: 200 overnight at 4°C and incubated with anti-c-Fos (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The sections were then incubated with biotinylated secondary antibody (1: 1000) for 1 hour. After the wash step with PBS, streptavidin-HRP was applied. Finally, the sections were rinsed with PBS and developed with a diaminobenzide dehydrochloride (DAB) substrate for 10 minutes. At least three random fields per section were checked at × 100.
All values were presented as mean ± S.E. Significant differences among groups were statistically analyzed using one-way analysis of variance (ANOVA) and non-parametric post Tukey test. All p values were double-tailed, and significance was set at P<0.05. All statistical analysis was performed using SPSS for Windows.
Results
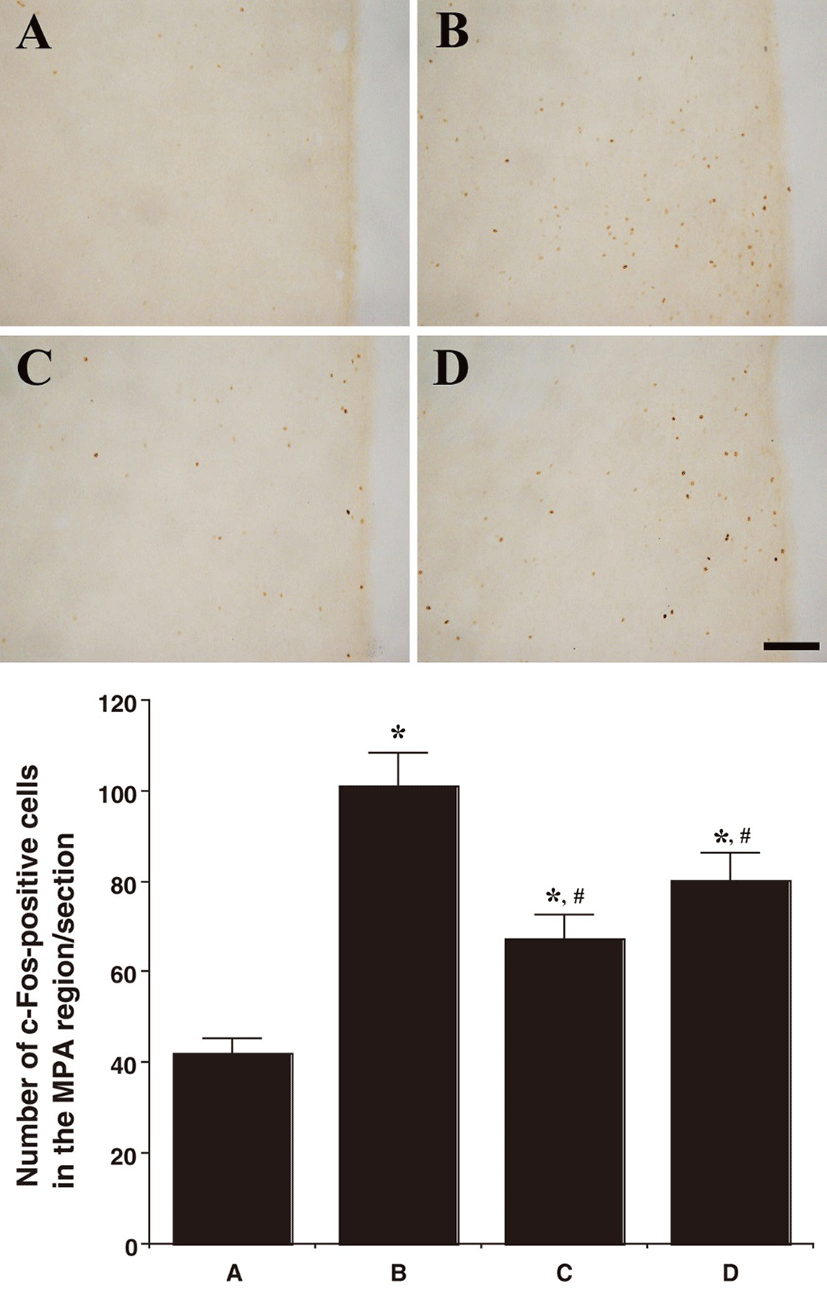
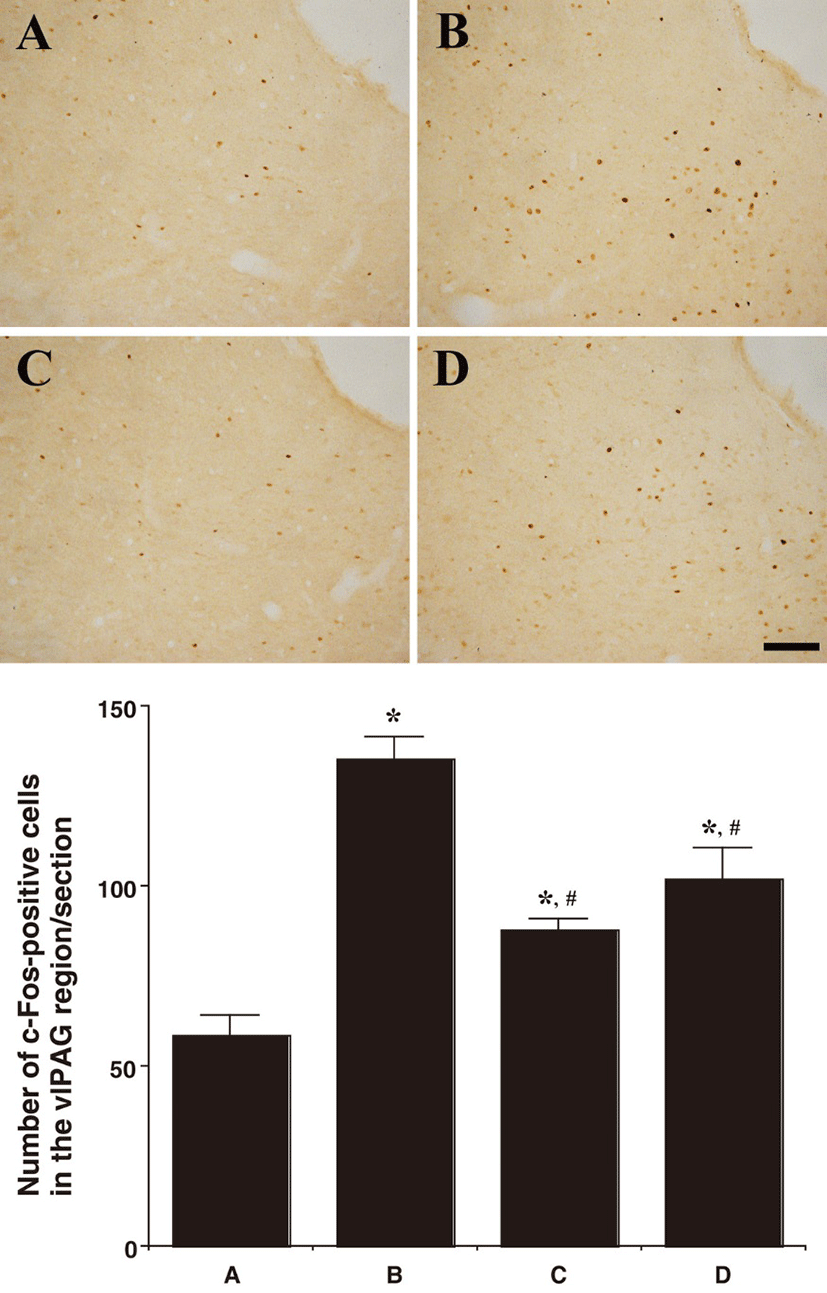
Fig 1, 2, and 3 showed photomicrographs of c-Fos-positive cells in the neuronal voiding centers of the brain (MPA, vlPAG, and PMC). In the MPA region, the number of c-Fos-positive cells was 42.00 ± 3.38/section in the control group, 101.16 ± 7.29/section in the BPH-induced group, 67.58 ± 5.15/section in the BPH-induced and P. ginseng-treated group, and 80.25 ± 6.18/section in the BPH-induced and finasteride-treated group. In the vlPAG region, the number of c-Fos-positive cells was 58.50 ± 5.57/section in the control group, 135.16 ± 6.22/section in the BPH-induced group,87.66 ± 3.47/section in the BPH-induced and P. ginseng-treated group, and 102.00 ± 8.78/section in the BPH-induced and finasteride-treated group.
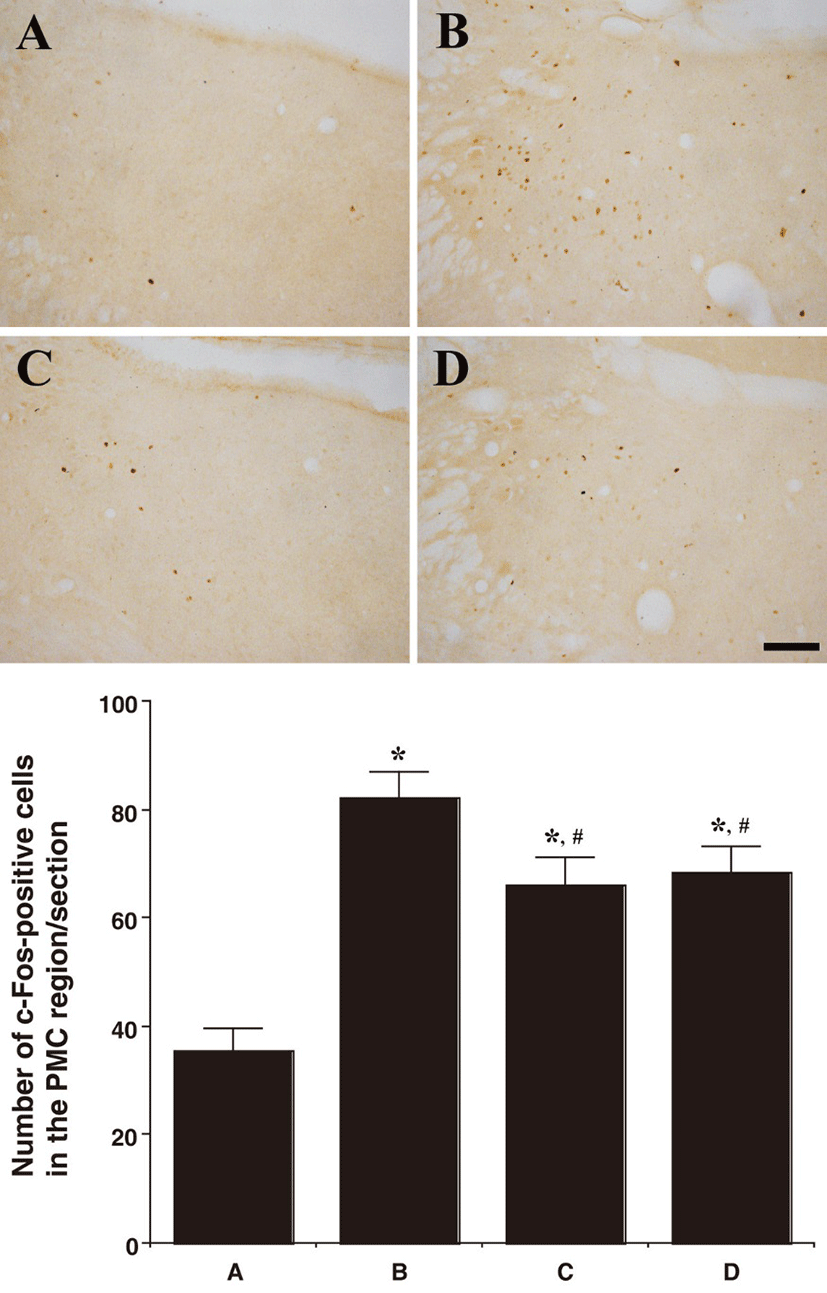
In the PMC region, the number of c-Fos-positive cells was 35.66 ± 3.82/section in the control group, 82.33 ± 4.69/section in the BPH-induced group, 66.00 ± 5.11/ section in the BPH-induced and P. ginseng-treated group, and 68.50 ± 4.78/section in the BPH-induced and finasteride- treated group.
In summary, the c-Fos expression in the neuronal voiding centers (MPA, vlPAG, and PMC) was increased by the induction of BPH (P<0.05) and P. ginseng treatment significantly decreased the BPH-induced c-Fos expression in the neuronal voiding centers (MPA, vlPAG, and PMC) (P<0.05).
Discussion
The results of this study showed that c-Fos expression in PMC, MPA, and vlPAG regions of brain was increased in the BPH group compared to those of the normal group, whereas the increased c-Fos expression in PMC, MPA, and vlPAG was significantly attenuated in finasteridetreated. In P. ginseng-treated group, the c-Fos expression was statistically significantly decreased similarly to the group treated with the positive drug.
In recent study [27] of P. ginseng, they showed that administration of P. ginseng in the testosterone induced BPH rat model significantly prevent prostate enlargement. Their observation in the study revealed that decreased expression of alpha-1D adrenergic receptor (Adra1d), EGFR, and BCL2 correlate with the protective effect of P. ginseng [27]. In addition to previous study, results of this study suggest biologic evidences that P. ginseng may have a protective effect on development of LUTS, therefore supporting the claim that P. ginseng helps ameliorate BPH symptoms.
The correlation of c-Fos expression and neuronal activation of central micturition centers in LUTS rat model was reported by Cho et al [29]. They reported that altered c-Fos expression is associated with neurogenic lower urinary tract dysfunction caused by intracerebral hemorrhage. Additionally, another study by Chung et al. [20] showed that c-Fos expressions in PMC, vlPAG, and MPA were correlated with stress urinary incontinence.
These evidences are related to bladder activity and c-Fos in brain [19, 22-25], however, they may partially support our result, because LUTS in BPH are caused by bladder outlet obstruction [30], and bladder obstruction is related to spinal cord c-Fos expression [31]. It may suggest that c-Fos is related to stress in bladder, which also occurs in LUTS due to BPH.
Moreover, overactive bladder is associated with BPH in approximately 40–75% of cases [32]. It is a very common symptom, and these two conditions share common bladder storage problems [33, 34]. Thus, these previous reports also support our observation that attenuated expression of c-Fos in P. ginseng treated BPH group may represent the decreased severity of LUTS in rats with BPH.
In summary, P. ginseng, which has a protective effect against BPH, may also have protective effect against LUTS, because c-Fos expression was increased in micturition centers of rats with BPH, and P. ginseng significantly decreased the c-Fos expression. Therefore, the present study suggests that P. ginseng could be used as an effective treatment for BPH.







