Introduction
Carcinogenesis is considered to be a multi-stage process that may arise as a result of DNA damage and clonal expansion of preneoplastic cells. Stem cells with a loss of DNA repair capacity have been associated with malignant transformation [1] and the formation of cancer stem cells [2]. It has also been reported that a distinct subpopulation of cancer stem cells might support several solid tumors [3].
This study was prompted by assumption that stem cells might have a significant role in hepatocarcinogenesis. We based this assumption on the following concepts: cell proliferation at the time of carcinogen exposure may be important for the fixation of genotoxic injury [4]; diethylnitrosamine (DEN) treatment in young animals induced a higher incidence of liver tumors compared to aged ones [5]; and hepatocellular and ductal carcinomas have been shown to originate from hepatic stem cells [6].
Our previous in vitro study reported that induction of Neighbor of Punc E 11 (Nope) expression was high in immature cells, such as embryonic stem cells and hepatic progenitor cells, but, not high in mature hepatocytes [7]. Considering that embryonic stem cells have self-renewal ability and may be differentiated into lineage-specific cell types [8], it seemed that cellular maturity might be important for expression of stem cell markers in response to a carcinogen.
Several stem cell markers, such as epithelial cell adhesion molecule (EpCAM) and Nope, have been implicated in hepatocarcinogenesis [9]. EpCAM is a membrane glycoprotein that is highly expressed on most cancer cells and a candidate stem cell maker [10]. Our previous study also reported that EpCAM expression was increased in DEN-induced hepatic tumor cells and was associated with cell proliferation [11]. Nope was a specific cell surface marker of stem/progenitor cells in the murine fetal liver that was also expressed in hepatocellular carcinoma [12], and high level of Nope expression has been observed at the time of transformation from preneoplastic lesion to malignant hepatocellular carcinoma [13].
Ataxia telangiectasia mutated (ATM) is one of markers for DNA double-strand breaks [14]. In a previous study, ATM knockout mice remained refractory to DEN-induced carcinogenesis [15]. However, it is not clear that DEN treatment can induce ATM expression in normal hepatocytes.
A recent study reported that DEN treatment induced an increase of cell proliferation in mouse hepatocytes, alpha mouse liver 12 (AML-12) cells cultured over a long-term period, by up-regulation of polymerase III-dependent gene transcription [16]. However, DEN effect on cell proliferation, DNA damage and stem cell marker(s) expression have been largely unknown in AML-12 cells cultured over a short-term period. Therefore, the purpose of the present study was to examine the cell proliferation, DNA damage, and EpCAM and Nope expression in AML-12 cells treated with DEN for 24 h.
Materials and Methods
AML-12 cells were purchased from ATTC (Manassas, VA) and were suspended in F12/Dulbecco’s Modified Eagle's Medium (DMEM) (Gibco-BRL, Gaithersburg, MD) supplemented with 10% (v/v) fetal bovine serum, 5 ng/mL insulin, 0.05 mg/mL transferrin, 5 ng/mL selenium, and 40 ng/mL dexamethasone. Cells were plated onto culture dishes at a density of 5 × 104 /cm2. The medium was changed every 3 days after seeding.
The MTT assay was used to quantify the proliferation of cells treated with DEN (Sigma-Aldrich, St. Louis, MO). Cells were seeded onto 96-well plates at a density of 2 × 104 /well for 24 h and then treated with DEN (25-800 μg/ mL) for 24 h. A 10 μL aliquot of MTT reagent 1 was added to each well. The plate was incubated for 4 h at 37°C until purple formazan crystals developed. Then, 100 μL of MTT reagent 2 was added to each well. After an overnight incubation, the absorbance was read at 595 nm with a spectrophotometer in four wells per treatment group.
Expression and distribution of ATM in AML-12 cells were determined by indirect immunofluorescence microscopy. Briefly, AML-12 cells were fixed with 3.5% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. The cells were permeated and blocked with 0.1% Triton X-100 and 5% fetal calf serum in PBS for 30 min. The fixed cells were washed and incubated for 1 h with an antibody (10 g/mL) against ATM. The cells were washed, incubated with fluorescein- conjugated secondary antibodies for 30 min, and observed under a fluorescence microscope.
Whole cell lysates were prepared by extracting proteins using a buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% sodium dodecyl sulfate (SDS), supplemented with protease inhibitors (10 g/mL leupeptin, 10 g/mL pepstatin A, 10 g/mL aprotinin, 1 mM 4-[2-aminoethyl] benzenesulfonyl fluoride, and 1 mM NaF and 1 mM Na3VO4 as phosphatase inhibitors).
The proteins were size-fractionated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes. Non-specific sites were blocked with Trisbuffered saline containing 3% non-fat dry milk and the membrane was incubated with the following antibodies: EpCAM (Abcam, Cambridge, MA) and Nope (Santa Cruz Biotechnology Inc., Santa Cruz, CA). The blots were washed, and proteins were visualized using the enhanced chemoluminescence method.
Results
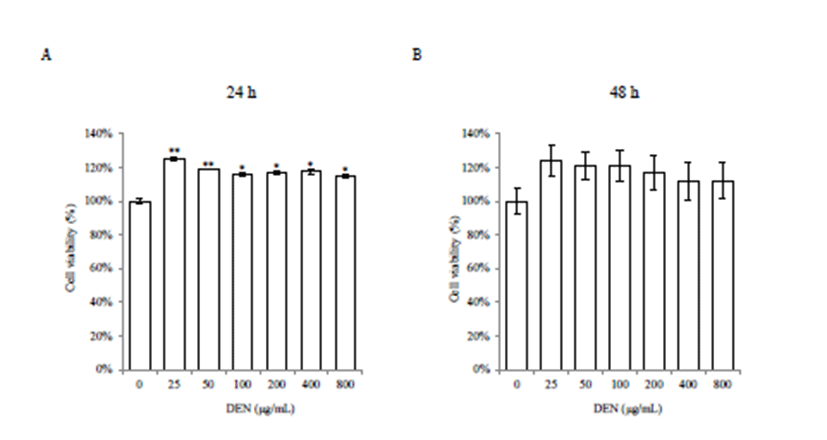
Various doses of DEN were used to assess the cytotoxicity in AML-12 cells. Cell morphology indicated that DEN treatment induced no cytotoxicity. Cell viability was significantly increased in all doses of DEN at 24 h (p<0.05 or p<0.01) (Fig. 1A). However, there was no significant increase at 48 h, even though it showed increased trend (Fig. 1B).
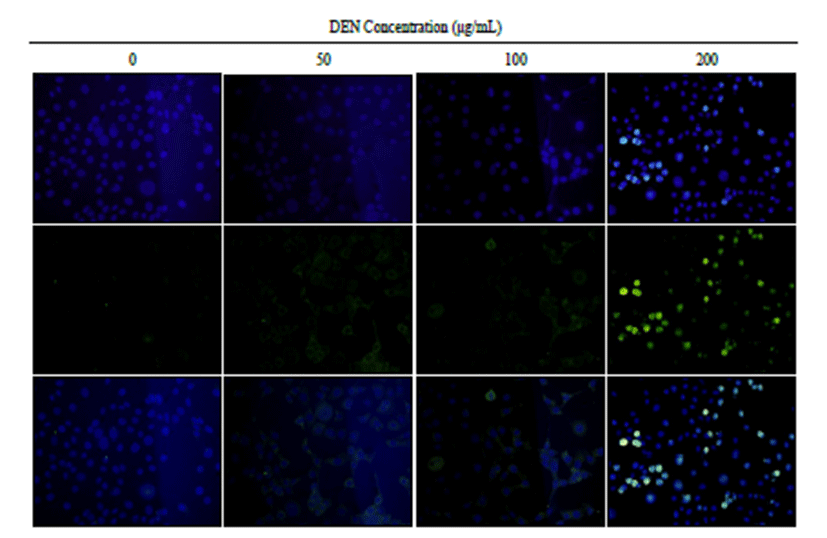
Immunofluorescence staining of ATM showed that there was an increase of ATM expression at doses of 50, 100 and 200 μg/mL of DEN treatment (Fig. 2).
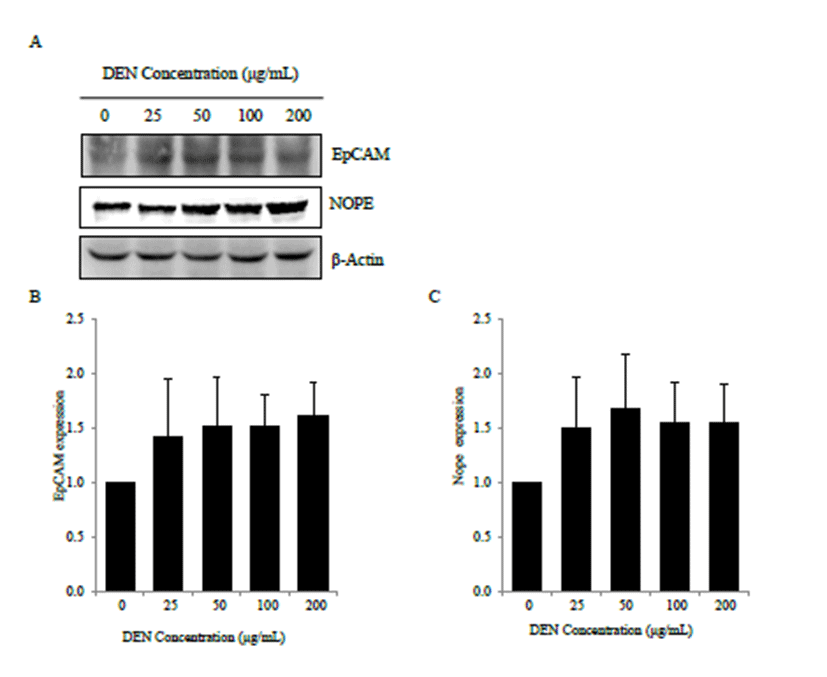
Western blot analysis showed that DEN treatment modulated EpCAM and Nope expression in AML-12 cells (Fig. 3A). Compared with control cells, DEN treatment showed increased trend of EpCAM expression (Fig. 3B). And DEN treatment also showed increased trend of Nope expression compared with control cells (Fig. 3C). At 48 h after DEN treatment, it also showed similar expression pattern compared to 24 h (data not shown).
Discussion
In this study, DEN treatment significantly increased cell proliferation in AML-12 cells, and ATM expression was dose-dependently featured in DEN-treated cells. Considering that DEN was metabolized by liver microsomes enzymes [17], became bioactive [18], and induced apoptosis in the early phase of hepatocarcinogenesis in vivo [19], and that apoptosis could promote early tumorigenesis [20] and generate potent growth-promoting signals [21], we considered that apoptosis of hepatocytes induced by a carcinogen could be associated with increased proliferation. Therefore, it seemed that increased proliferation in AML-12 cells might be associated with increased apoptosis in early time of carcinogen exposure. Moreover, DEN exposure could elicit an inflammatory response in nonparenchymal cells, resulting in compensatory proliferation of quiescent hepatocytes [22]. However, DEN treatment did not result in increased cell proliferation of HepG2 cells in our study (unpublished data). It seemed it might be originated from cellular difference and response potential to carcinogens. Even though the utilization of cultured hepatocytes is popular in biological studies [23], immortalized cell lines also have an inherent disadvantage owing to different metabolic and functional properties. Further studies are warranted to investigate the occurrence and/or persistence of DNA damage in different hepatic cell lines induced by DEN treatment.
In this study, DEN treatment showed increased trend of EpCAM and Nope expression in AML-12 cells. Previous study reported that EpCAM expression was slightly increased in both immature and mature mouse hepatocytes treated with DEN [24]. However, Nope expression was highly expressed in immature mouse hepatocytes treated with DEN compared to mature ones [7]. Further studies are warranted to investigate the different expression level of stem cell markers in several hepatic cell lines.
Taken together, DEN treatment increased cell proliferation in AML-12 cells, and it was associated with increased of ATM expression and modulated expression of EpCAM and Nope.







