The common honey bee belongs to the species Apis mellifera L. and participates in some activities that are closely related to human beings. Since ancient times, it has been believed that the honey bee venom (BV) can be used to treat certain immune-related diseases, such as arthritis and rheumatic conditions [1-3]. BV comprises several components, including cell-lytic peptides, diverse enzymes, and bioactive amines. The two major proteinaceous components of BV are melittin, a 26-amino acid cationic cell-lytic peptide, and phospholipase A2 (PLA2), an enzyme that hydrolyzes membrane phospholipids to produce lysophospholipids and arachidonic acid [4]. PLA2 isolated from BV (bvPLA2) belongs to the two notable families with a common enzymatic activity. It is well known that PLA2 can induce an allergic response upon entering the body through bee or wasp stings [5]. However, PLA2 also displays antibacterial and anticoagulant actions and plays an active role in anti-inflammatory processes [6-8]. Recently, it was revealed that PLA2 has protective immune responses against several diseases including asthma, Parkinson’s disease, Alzheimer’s disease, and drug-induced organ inflammation [9,10]. Moreover, bvPLA2 is sensed by the innate immune system and induces a receptor ST2-dependent primary type 2 immune response [11]. And also, PLA2, which is isolated from the venom of Crotalus durissus terrificus, inactivates Dengue and yellow fever viruses, as well as other enveloped viruses by disrupting the viral envelope [12]. However, the underlying mechanisms of antiviral activities by bvPLA2 have not been fully understood. Therefore, we investigated whether bvPLA2 inhibits viral replication by other specific mechanism.
The PLA2 was isolated from BV (Chung Jin Biotech Corporation, Korea) using high performance liquid chromatography (HPLC) instrument consisting of Waters 2525u binary HPLC pump, Waters 996 photodiode array detector, Waters 717 plus and Waters Fraction Collector III. Chromatography was conducted on a Phenomenex Jupiter 10u Preteo 90A Column (250 X 21.20 mm), and 1,000 μL (10 mg/mL) of BV was injected. The mobile phase consisted of 0.2% TFA in water (Solvent A) and 0.22% TFA in acetonitrile (Solvent B). The gradient mode was as follows: 0 min 100% A and 0% B; 25 min 50% A and 50% B; 60 min 100% A and 0% B. The flow rate was 8.0 mL/min, and the elution was monitored at 220nm. Collected fractions were compared with standard PLA2.
The inhibitory activity of bvPLA2 was tested in HEK293T, HeLa, Vero, and MDCK cells (12 well plates: 5×105 cells/well) with diverse RNA and DNA viruses which can express the green fluorescent protein (GFP) by using the attachment assay. Briefly, for the attachment assay, viruses and bvPLA2 (2.0 or 3.0 μg/mL) were simultaneously added into the cells and incubated with the cells for 1 hr at 4ºC. After washing, the cells were transferred to 37ºC. At 24 hour post infection (hpi), cells were harvested to measure the GFP absorbance (the cytopathic effect for EV-71) (data not shown), and supernatants (with and without cells) were collected for a standard plaque assay of the viruses (Fig. 1).
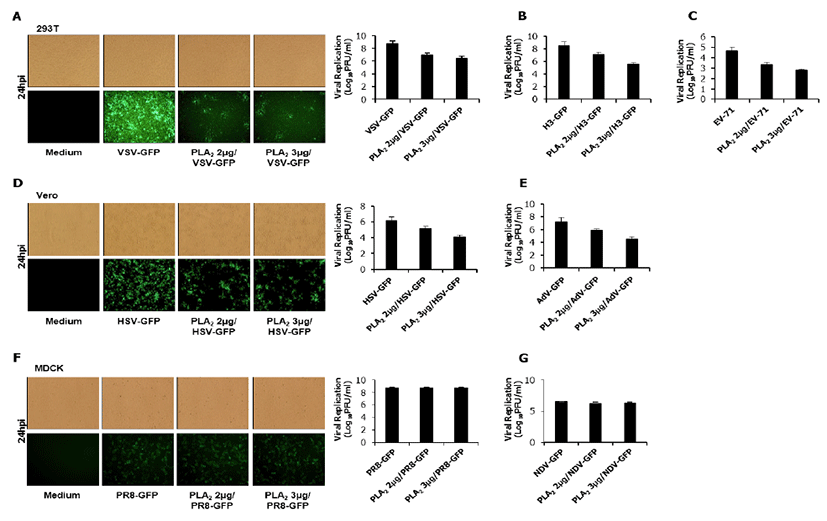
As shown in Fig 1, bvPLA2 inhibited the replication of Vesicular Stomatitis virus (VSV-GFP, Fig.1A), Coxsackie virus (H3-GFP, Fig.1B), Enterovirus-71 (EV-71, Fig.1C), Herpes Simplex virus (HSV-GFP, Fig.1D) and Adenovirus (AdV-GFP, Fig.1E). However, bvPLA2 couldn’t inhibit the replication of Influenza A virus (PR8-GFP, Fig.1F) and Newcastle disease virus (NDV-GFP, Fig.1G). These results suggested that bvPLA2 can block the attachment of diverse virus using specific cell receptors.
The binding of viruses to their target cells is the initial step in establishing an infection. Numerous viruses utilize specific or non-specific cellular receptors to infect target cells. A full spectrum of cell surface molecules, including glycoproteins, glycolipids, and proteoglycans participate in cell–virion interaction and recognition. Recent studies have revealed that heparan sulfate (HS) (Sigma, Korea) plays a role in facilitating viral infection through an interaction with viral proteins [13]. HS glycosaminoglycans also serve as receptors for the infection of Herpes Simplex virus (HSV), Adenovirus (Adv), Respiratory Syncytial virus (RSV), and Hand, Foot and Mouth disease virus [14-17]. However, the hemagglutinin protein of the Influ enza virus and the hemagglutinin-neuraminidase protein of the Newcastle disease virus (NDV) interact with sialic acid as a cellular receptor to form virus-to-cell binding complexes [18, 19]. On the basis of these previous reports and our results, we assumed that cellular HS might be a target of bvPLA2.
First, we checked whether HS which acts as a cellular receptor could affect HSV-to-cell interaction. As shown in Fig. 2A and 2B, HS has an inhibitory effect on the attachment of HSV to the cell surface dose dependently, which was inconsistent with bvPLA2 treatment. These data suggest that treated HS acts competitively with cellular HS to interact with virions. In order to evaluate whether bvPLA2 binds to HS on the cell surface, we performed an attachment assay of HSV through co-incubation with bvPLA2 and HS (Fig. 2C).
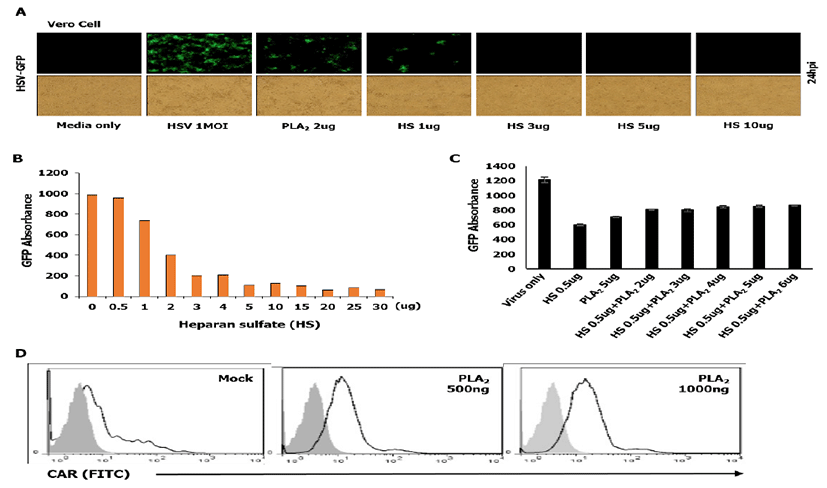
Dose-dependent bvPLA2 and HSV-GFP were incubated in the cells with HS (0.5ug) for 1 h at 4ºC. After washing, the cells were transferred to 37ºC, and GFP absorbance was measured at 24 hpi. As shown in Fig. 2C, bvPLA2 or HS inhibited the viral replication. However, in case that bvPLA2 was added to the cells with HS, viral replication did not increase dramatically compared with that in cells treated only with HS. These results suggest that bvPLA2 inhibits viral replication not by blocking HS but by blocking some other receptors on the cell surface.
The coxsackie virus and adenovirus receptor (CAR) is a receptor for the group B Coxsackie virus and subgroup C Adenovirus [20]. Therefore, we investigated whether bvPLA2 binds to CAR by a fluorescence-activated cell sorting (FACS) analysis. Vero cells were treated with bvPLA2 in a dose-dependent manner for 1 h and fixed with paraformaldehyde (PFA). Then, the cells were incubated with an FITC-conjugated CAR antibody (Thermo-Fisher, Korea) and subjected to FACS analysis. As shown in Fig. 2D, no change was observed in bvPLA2-treated cells compared with that in mock-treated cells.
In this study, although we could not demonstrate the exact mechanism how bvPLA2 blocks the cellular receptors involved in viral attachment, we found that it inhibits the replication of some viruses by blocking their attachment to the cell surface. Further studies are warranted to determine the specific target receptors of bvPLA2 that block the attachment of viruses and the reason why bvPLA2 did not inhibit the replication of PR8 and NDV. However, our observations suggest the possibility that bvPLA2 could be used as a prophylactic antiviral reagent against some RNA and DNA viruses.







