Introduction
The incidence of multiple primary malignancies is increasing, not only as a result of aging of the population, but also as a result of advances in diagnostic technology [1-3]. However, it is difficult to diagnose a malignant lesion that is considered to be a metastasis in a patient with a pathologically-confirmed malignancy when that lesion arises not as metastasis from the underlying malignancy, but from the synchronous presentation of a double primary malignancy.
Here, we report a rare and clinically meaningful case of synchronous double primary duodenal adenocarcinoma and renal cell carcinoma (RCC), which initially was diagnosed as metastatic duodenal adenocarcinoma in lungs, liver, and kidney.
Case Report
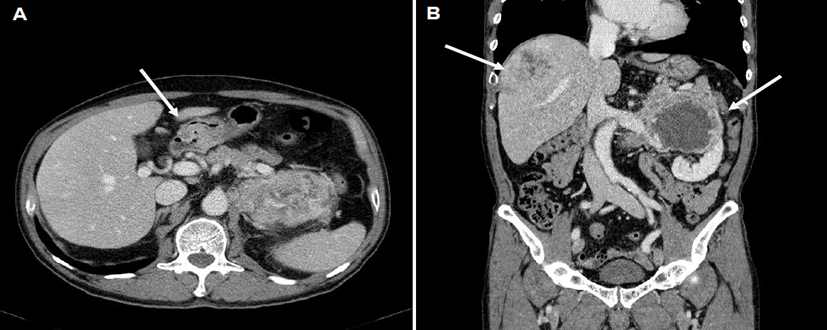
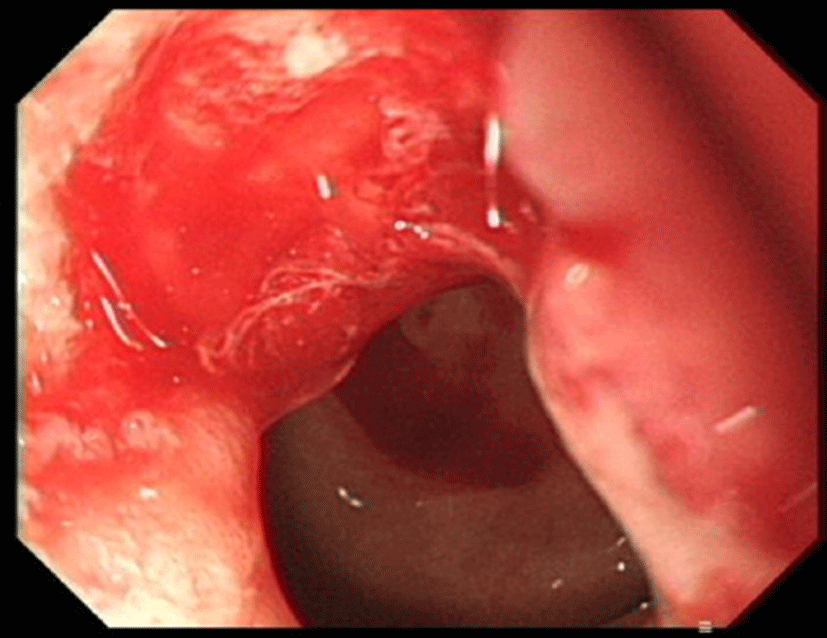
A 66-year-old man presented with a 3-week history of a progressively enlarging palpable abdominal mass. An abdominal examination revealed a deep-seated, firm, non-tender, 5 cm sized mass in the left upper quadrant. Computed tomography (CT) of the chest revealed multiple small nodules in both lungs. CT of the abdomen revealed thickening of the duodenal wall (Fig. 1A), a 7.5 cm sized heterogeneous enhancing hypervascular mass in the right lobe of the liver and a 12 cm sized well-marginated exophytic mass with internal necrosis in the left kidney, which invaded the adrenal gland and psoas muscle (Fig. 1B). However, percutaneous needle biopsy of hypervascular mass in liver or kidney initially cannot be performed because of extremely high bleeding risk. Esophagogastroduodenoscopy revealed an ulcerative lesion in the pyloric channel and duodenal bulb (Fig. 2), and endoscopic biopsy of this mass resulted in a histologic diagnosis of moderately-differentiated adenocarcinoma, based on the morphology visualized with hematoxylin and eosin (H&E) staining. He was initially diagnosed with duodenal adenocarcinoma with multiple metastases in the lungs, liver, and kidney. He was treated with capecitabine and oxaliplatin as first line palliative chemotherapy for 6 months, followed by two months of 5-fluorouracil, leucovorin, and irinotecan as second line palliative chemotherapy.
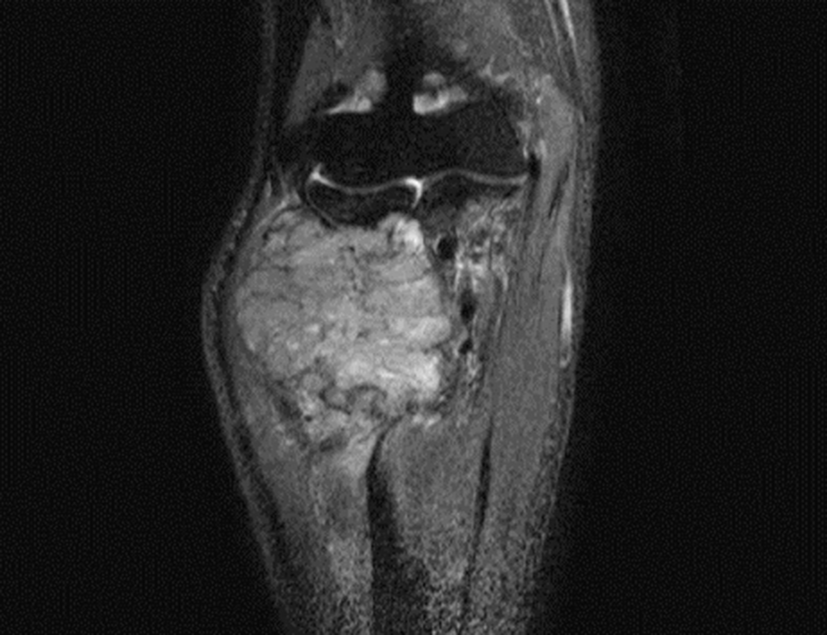
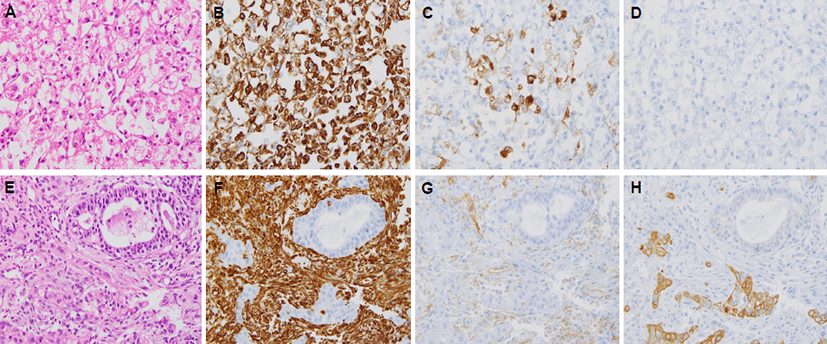
He developed a progressively enlarging mass in the right forearm after 10 months of initial diagnosis and a firm, fixed, non-tender, 8 cm sized mass in the right forearm. Magnetic resonance imaging (MRI) scan of the right forearm revealed a 6 cm sized bone-destructive hypervascular mass in the right proximal radius (Fig. 3). An incisional biopsy was performed. The tumor cells were polygonal with clear cytoplasm and vesicular nuclei with prominent nucleoli by H&E staining (Fig. 4A). Immunohistochemical staining was positive for vimentin (1:200; Dako, Glostrup, Demark) (Fig. 4B) and focally positive for cluster of differentiation (CD) 10 (1:100; Leica, Newcastle-upon-Tyne, UK) (Fig. 4C), but negative for cytokeratin 7 (1:600; NeoMarkers, Fremont, USA) (Fig. 4D) and cytokeratin 20 (1:150; Dako, Glostrup, Denmark). A diagnosis of metastatic RCC was made based on the histological and immunohistochemical findings. We re-examined the material from the patient’s previously biopsied duodenal tumor. The microscopic findings upon H&E staining of the duodenal tumor differed completely from those of the forearm mass, consistent with a histological diagnosis of moderately-differentiated adenocarcinoma (Fig. 4E). Immunohistochemical staining showed that duodenal tumor cells were entirely negative for vimentin (1:200; Dako) (Fig. 4F) and CD 10 (1:100; Leica) (Fig. 4G), but focally positive for cyto keratin 7 (1:600; NeoMarkers) (Fig. 4H). Based on these results, the tumor in the left kidney was considered not to be a metastatic duodenal adenocarcinoma, but a primary RCC, and therefore the final diagnosis was synchronous double primary duodenal adenocarcinoma and RCC. The patient was treated with palliative radiotherapy to the mass in the right proximal radius for pain control, followed by sunitinib to treat the metastatic RCC. However, multiple malignant lesions of lung, liver, and kidney were aggravated, and he died of progressive disease 19 months after initial diagnosis.
Discussion
Primary duodenal adenocarcinoma is a rare malignant neoplasm representing approximately 0.5% of all gastrointestinal cancers [4-6]. The most common metastatic sites for duodenal adenocarcinoma are the liver, lung, retroperitoneum, and peritoneal carcinomatosis [7-8]. In the described case, multiple malignant lesions were observed in the lung and liver, which are common metastatic sites for duodenal adenocarcinoma, and in the kidney, which also is a common site of metastases, with a reported incidence of 2–20% at autopsy [9]. For that reason, he was diagnosed initially with duodenal adenocarcinoma with multiple metastases in the lungs, liver, and kidney.
However, all the malignant lesions in liver and kidney in this case appeared as highly enhancing, hypervascular tumors on contrast-enhanced CT. In addition, the mass that appeared 10 months later in the right proximal radius was also hypervascular on MRI scan. Hypervascular masses are seen with hepatocellular carcinoma, RCC, melanoma, sarcoma, and neuroendocrine carcinoma [10-11]. Therefore, hypervascular masses in the liver and kidney that are initially considered metastases from duodenal adenocarcinoma must be considered not only as possible hypervascular metastases, but also as possible synchronous double primary hypervascular tumors. Furthermore, previous studies described a wide spectrum of differentiating charactecistics between primary and secondary renal masses [9, 12-14]. Primary RCC is single, unilateral, nonwedge-shaped, hypervascular, exophytic, and easily transgresses the renal capsule, but renal metastases are characterized as small, multiple, bilateral, wedge-shaped, hypovascular, and less exophytic, and located within the renal capsule. The renal mass in our presented case was 12 cm in size, single, unilateral, nonwedge-shaped, hypervascular, and exophytic, a pattern consistent with primary RCC. A diagnosis of metastatic RCC was confirmed histologically on slides stained with H&E staining and a panel of antibodies, which included probes recognizing antigens specifically expressed by RCC. Clear cell RCC expresses a variety of low molecular cytokeratins, vimentin, and CD10 [15-16]. The tumor cells with clear cytoplasm obtained from the hypervascular mass in the right proximal radius is positive for vimentin and CD10, but negative for cytokeratin 7, whereas duodenal adenocarcinoma is positive for cytokeratin 7, but negative for vimentin and CD10. Therefore, the differential diagnosis of duodenal adenocarcinoma and RCC was made based on the morphologic and immunohistochemical findings.
Synchronous double primary duodenal adenocarci noma and RCC are very rare; to the best of our knowledge, only two cases have been reported in the English language literature [17-18]. Our case has several unusual features compared with those of previous reports of synchronous double primary duodenal adenocarcinoma and RCC. In our case, the patient initially presented with a palpable abdominal mass associated with RCC. Furthermore, the previously reported two patients underwent surgical resection of the duodenal adenocarcinoma and renal mass because there were no other metastatic lesions present, after which a pathologic confirmation of RCC was made by examining the surgical specimen. However, our case presented with multiple malignant lesions in the lungs, liver, duodenum, and kidney, and the pathologic diagnosis of RCC was made only after a mass in the right proximal radius appeared after 10 months, on which an incisional biopsy was performed. Surgical resection is considered the standard of care in patients with renal masses thought to be RCC [19-20]. Moreover, survival in patients with oligometastatic renal disease is better in patients who undergo surgical resection of the kidney [9]. Therefore, renal surgery along with surgical resection of the pathologically-confirmed malignancy should be considered in cancer patients with a single mass in the kidney. Moreover, although a cancer patient may have multiple malignant lesions, including a renal mass, which could be considered metastases, the clinician should consider fine-needle aspiration or biopsy of the renal mass if it is highly suggestive of primary RCC although bleeding risk according to the needle biopsy is high.
This report describes a rare case of synchronous double primary RCC initially diagnosed as metastatic duodenal adenocarcinoma. Patients with a pathologically confirmed malignancy with lesions suggestive of other types of malignancy are at risk for being diagnosed with metastasis from the underlying malignancy. Furthermore, misdiagnosis of synchronous double primary malignancy as a metastasis may allow the underlying disease to progress without proper local or systemic therapy. Thus, a high index of suspicion, a careful review of the imaging, and biopsy of metastatic lesions are necessary if metastatic lesions show unusual metastatic patterns or unusual clinical presentations.







