Introduction
Kodamaea ohmeri, previously known as Pichia ohmeri or Yamadazyma ohmeri, is an ascosporogenous yeast that belongs to the Saccharomycetaceae family. K. ohmeri is a teleomorph of Candida guilliermondii var membranifaciencs, and is an environmental strain commonly used in the food industry to ferment pickles [1]. However, it is also an emerging opportunistic pathogen in immunocompromised patients worldwide [2, 3]. K. ohmeri has been implicated in fungemia [4-7], peritonitis [8], endocarditis [9, 10], fungiuria [11, 12], and cellulitis [13]. In South Korea, a few K. ohmeri infections have been reported, and most cases were caused by central venous catheter [14]. In one case, insertion of a stent in colon caused the fungemia [15]. Here, we report a case of K. ohmeri fungemia that occurred after endoscopic retrograde cholangiopancreatography (ERCP) and insertion of biliary stent in the patient with cancer of the ampulla of Vater. He was successfully treated with fluconazole.
Case report
A 70-year-old man was admitted to emergency department for abdominal pain and vomiting. He had been diagnosed with carcinoma of the ampullar of Vater 2 months earlier and had undergone placement of a biliary stent. On admission, he had a temperature of 36.7°C, pulse rate of 80/min, respiratory rate of 20/min, and blood pressure 120/80 mmHg. No neurological deficits were detected. On hospitalization day 11, he underwent ERCP to resolve obstruction of the ampullar of Vater by inserting a biliary stent. The next day, his temperature was 38.5°C. The neutrophil (%) and C-reactive protein (CRP) were elevated (71.5% and 4.66 mg/dL). Blood samples for culture were obtained from a peripheral vein. Considering bacteremia caused by invasive procedure (ERCP and stent insertion), the patient was treated empirically with cefotaxime for 3 days but remained febrile. On hospital day 15, cefotaxime was replaced with fluconazole (for 14 days) after the yeast has been identified as K. ohmeri with a minimal inhibitory concentration (MIC) of 2 μg/mL. Subsequent two blood cultures were negative. The neutrophil count was normalized (57% of WBC), and CRP decreased to 2.3 mg/dL. In addition, the level of alkaline phosphatase and γ-Glutamyl transferase decreased to 314/257 IU/L from 742/324 IU/L after receiving percutaneous transbiliary drainage procedure to resolve obstruction of biliary tract. The patient was discharged in a stable condition on hospital 35 days.
The microbiologic findings of this case were as follows. There was a positive growth 24 h after the blood cultures. Gram staining of blood culture indicated yeast forms with budding shapes that suggest Candida spp. (Fig. 1). Gray-white colonies were observed 18 h after subculture on blood agar plates, and no germ tube formation was detected (Fig. 2.). The biochemical reaction on a Vitek YST card (bioMérieux, France) indicated a 99% probability of K. ohmeri with bionumber of 4544557045341370. The yeast was identified as K. ohmeri based on the results of biochemical tests and sequencing analysis of the D1/ D2 domains of the 26S rRNA gene and the internally transcribed spacer (ITS) region. The 26S rRNA gene and ITS sequence of the isolates were identical to those of K. ohmeri (GenBank accession No. FJ215866.1). Antifungal susceptibility testing was performed with the Vitek AST-YS06 card (bioMérieux, France), and the MICs of the antifungal agents for this yeast were as follows: amphotericin B 0.5 μg/mL: fluconazole 2 μg/mL; voriconazole ≤ 0.12 μg/mL; flucytosine ≤ 1 μg/mL. According to the Clinical and Laboratory Standard Institute (CLSI) [16] that recommended breakpoint (in μg/mL) for Candida spp., the isolated K. ohmeri was sensitive to amphotericin B, fluconazole, voriconazole and flucytosine.
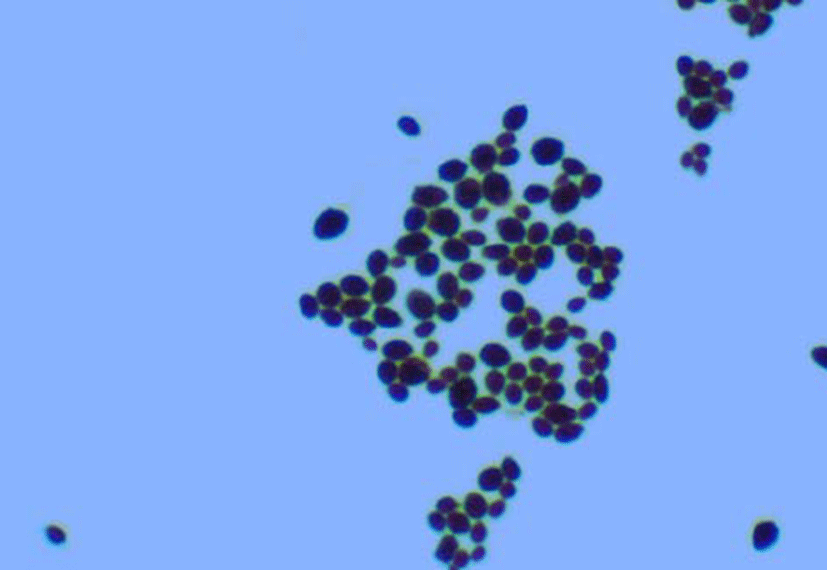
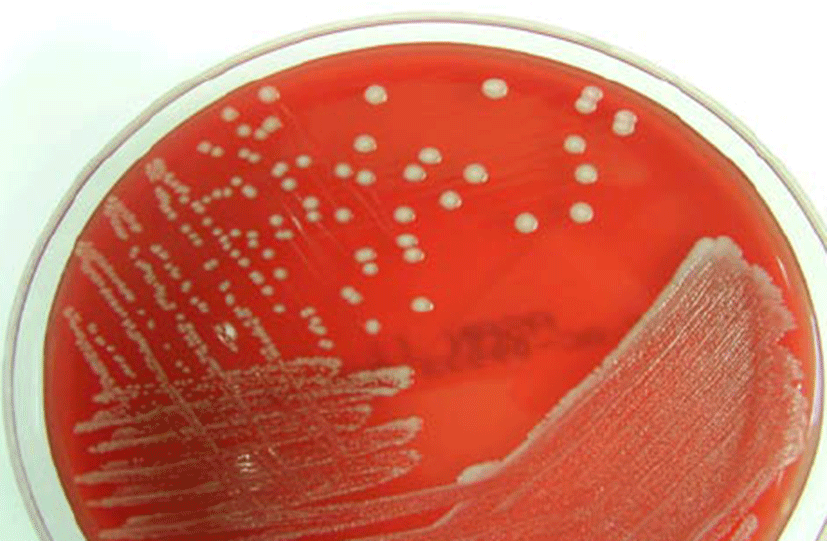
Discussion
K. ohmeri is an opportunistic yeast that is increasingly being found in immunocompromised patients worldwide. This report described an isolate of K. ohmeri from the patient with carcinoma of the ampulla of Vater following ERCP. The isolate was identified by biochemical methods. As K. ohmeri is a rare opportunistic microorganism in humans, the isolate was further investigated using molecular methods. The sequencing analysis confirmed the biochemical identification, providing a useful method for the rapid identification of species in clinical specimens.
This case may represent a hospital-acquired infection, which result from ERCP and insertion of a biliary stent. Although K. ohmeri infection has rarely been reported as a nosocomial infection, it is considered as an important fungal pathogen emerging in humans, based on recent case reports (Table 1). This case report presents a pathogen occurring in an immunocompromised patient after a medical procedure.
Abbreviations: V-P, ventriculoperitoneal; KA, ketoacidosis; DM, diabetes mellitus; CRF, chronic renal failure; TOF, tetralogy of Fallot; VLBW, very low birth weight; GI, gastrointestinal; IP, invasive procedure; CVC, central venous catheter; UAVC, umbilical artery and vein catheter; Amp-B, amphotericin-B, FC, fluconazole; RC, recovered.
The identification of Kodamaea in hospital laboratories using conventional methods is not always possible. It may be reported as Candida tropicalis or Candida haemulonii by conventional methods [14]. CHROMagar Candida can be valuable for the provisional identification as the color of K. ohmeri colonies changes [14, 17]. Automated identification systems such as Vitek 2 ID YST or API 20 C are the mainstay of the laboratory identification of this rare fungus. In addition, molecular methods are used to confirm rare microorganisms such as K. ohmeri [14, 18]. PCR amplification was followed by sequencing of the 18S rRNA, D1/D2 domain of 26S rRNA, and internal transcriber spacer 1/2(ITS) of the ribosomal DNA. The pathogen in this case was correctly identified with the Vitek 2 ID YST and confirmed by 26S rRNA gene and ITS sequencing analysis.
Although there are no definitive superior antifungal agents, reports have suggested that amphotericin B and fluconazole are effective for the treatment of K. ohmeri infection [12, 19, 20]. The current treatment strategy for K. ohmeri infection includes the removal of medical devices (e.g., central venous and Foley catheter) and the use of effective antifungal agents. The mortality of K. ohmeri infection is 40% [4, 13], which suggests that K. ohmeri infection is critical or that treatment of this infection is difficult. Therefore, if fungemia is suspected in blood cultures of a immunocompromised patient, prompt therapy with an antifungal agent and removal of any predisposing factors are required. Our patient did not have a central venous catheter or medical device and the sign of fungemia developed after ERCP. Therefore, the procedure (ERCP and stent insertion) might have caused the fungemia. The patient was treated with antifungal agents without removing the stent and recovered from the infection.
In conclusion, this report provides another examples of K. ohmeri as an emerging infection agent in the immunocompromised patients and shows that molecular analysis is useful for species identification. To the best of our knowledge, this is the first report of the isolation of K. ohmeri in a patient who had recently undergone ERCP.







