Introduction
Hypertrophic cardiomyopathy (HCM) is the most commonly diagnosed feline myocardial disease and is characterized by hypertrophy of a non-dilated ventricle [1]. In humans, HCM is known as a familial disease and is the most common cause of sudden cardiac death in young people [2]. Feline HCM was first recognized in the 1970s [3] and still remains the major cause of cardiac morbidity and mortality as well as sudden death in cats [4-6]. Signs of congestive heart failure (CHF), arterial thromboembolism (ATE), and sudden death are common clinical features in affected cats [1]; however, a recent study reported HCM was also diagnosed in apparently healthy cats [7]. Several reports have described clinical features, prognostic factors, and survival times of feline HCM [1, 4, 5, 8]. The presence of clinical signs and an enlarged left atrial (LA) size are the most important negative prognostic factors, whereas other factors such as breed, increased heart rate, and age also affect survival time. Most of these prognostic factors and survival times of HCM have been researched in European countries [1, 4, 8] and United States [5]. The purpose of this retrospective study was to characterize the clinical presentation, diagnostic features, and outcomes (survival times) of cats diagnosed with symptomatic HCM in our country.
Materials and Methods
Cats admitted to emergency service and diagnosed with HCM between June 2009 and August 2013 were identified. Medical records were examined to verify the clinical diagnosis of HCM based on review of physical examination, blood pressure measurements, laboratory examination, as well as electrocardiographic, radiographic, and echocardiographic findings. Cats were included if diagnosis of HCM had been made based on 2D and/or M-mode echocardiography.
Cats diagnosed with hyperthyroidism, systemic hypertension (systolic blood pressure≥180 mmHg), other types of cardiac disease, and concurrent systemic disease were excluded from the study. Asymptomatic cats diagnosed with HCM were excluded from the study. Symptomatic HCM cats displaying both clinical signs attributed to aortic thromboembolism (ATE) and congestive heart failure (CHF) were also excluded from the study.
The medical records of each case were reviewed for age, sex, breed, body weight, heart rate, respiratory rate, blood pressure, temperature, clinical signs at presentation, presence of murmur or gallop rhythm, and time to hospital visitation. Cats were classified according to clinical signs at presentation. Cats presenting with any clinical signs attributed to aortic thromboembolism (ATE) were classified as “ATE”, and cats showing clinical signs associated with congestive heart failure (CHF) such as tachypnea, profound dyspnea, crackle, or muffled heart sound coupled with respiratory distress were classified as “CHF” [1]. The presence of aortic thrombus was confirmed by ultrasonography or autopsy. Electrocardiographic examination based on a 6-channel electrocardiogram (ECG; Cardiofax GEM ECG-9020K, Nihon Kohden, Tokyo, Japan) was reviewed for arrhythmia, and thoracic radiographs were reviewed to determine LA enlargement and pulmonary and/or pleural effusion. Complete echocardiographic examination results (Logiq400, GE Healthcare Medical Systems, Milwaukee, WI, USA), including transthoracic 2-D, M-mode, spectral, and color-flow Doppler, were also reviewed. Systolic and diastolic wall thicknesses of the left ventricle (LV) free wall (LVFWd and LVFWs, respectively) and interventricular septum (IVSd and IVSs, respectively) were measured in right parasternal 2-D and M-mode views. The primary diagnostic criterion of left ventricular hypertrophy was LVFWd or IVSd equal to or exceeding 6 mm [8]. Systolic and diastolic dimensions of the LV internal diameter (LVIDd and LVIDs, respectively) were measured in the same view, and fractional shortening (FS) was calculated [FS=(LVIDd – LVIDs)/LVIDd × 100]. Measurements of aortic (AO) and LA diameters were obtained in 2-D right parasternal short-axis view, and the LA/AO ratio was calculated. Diameter of the left atrium (LAD) was measured in right parasternal long-axis view, parallel with the mitral annulus [1, 10]. The presence or absence of systolic anterior motion (SAM) and thrombi was reviewed based on 2D and M-mode measurements [11].
For all cats, complete blood count (CBC) and serum chemistry results, including electrolytes, were evaluated. All medications during hospitalization or after discharge were recorded, and survival time was obtained by reviewing medical records and contacting owners.
The results are expressed as mean ± standard deviation (S.D.), and non-parametric data are expressed as median and range. Categorical data were compared with Fisher’s exact test. Variable continuous data were assessed by Student’s t-test and Mann-Whitney test as appropriate in each clinical group. Survival analysis was performed using the Kaplan-Meier method and Log rank test. Statistical significance was defined as P<0.05. SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA) and Excel 2010 (Microsoft, Redmond, WA, USA) were used to perform statistical analyses.
Results
Thirteen client-owned symptomatic HCM cats were included in this study. At initial presentation, variable clinical signs were detected (Table 1). Respiratory distress was detected in half of the cats (five cats, 38.5%). Among them, four cats showed pulmonary edema and/or hemorrhage, whereas one cat had pleural effusion. Non-specific clinical signs such as depression were commonly detected. Ten cats showing clinical signs related to ATE such as pelvic limb paralysis were classified as the ATE group. Two cats in the ATE group had concurrent CHF and were excluded from the study. Cats only showing CHF signs (five cats) were classified as CHF group. The median times for hospital visitation after initial clinical signs were 2 days (range 0.5~4 days) in the ATE group and 4.8 days (range 1~7 days) in the CHF group. Median age of cats with symptomatic HCM was 6 years (range 1.4~7 years). Approximately half of cats were male (53.8%), and all of them were castrated. Most common breeds were domestic short hair, followed by Turkish angora, Siamese, Persian, and Himalayan. Upon admission, mean heart rate was 182.5 ± 23.3 bpm, respiratory rate was 64.6 ± 31.4 /min, and blood pressure was 135.8 ± 8.5 mmHg. Heart murmur was detected in 33.3% of cats with symptomatic HCM. However, there was no significant difference between groups with regard to age, sex, or initial physical evaluation (Table 2).
Hematologic evaluation was also documented (Table 3). In the ATE and CHF groups, alanine transaminase (ALT, reference range 28~106 U/L), aspartate transaminase (AST, 12~46 U/L), creatine kinase (CK, 73~260 U/L), and lactate dehydrogenase (LDH, 46~350 U/L) levels were higher than normal. Especially, significant elevation of primarily muscle enzymes [AST (P=0.028); CK (P=0.038)], LDH (P=0.008) and glucose (P=0.013)] was observed in the ATE group. Blood urea nitrogen (BUN) and creatinine (CREA) values were elevated in the ATE group; however, there was no significant difference compared with the CHF group. Results of further hematological evaluation showed no significant differences between the two groups.
ATE; arterial thromboembolism, CHF; congestive heart failure, WBC; white blood cell, RBC; red blood cell, Hb; haemoglobin, PCV; pack cell volume, PLT; platelet, BUN; blood urea nitrogen, CREA; creatinine, ALT; alanine transaminase, AST; aspartate transaminase, CK; creatine kinase, LDH; lactate dehydrogenase, TP; total protein, ALB; albumin, Ca; calcium, P; phosphorus, K; potassium, Na; sodium, Cl; chloride.
Three cats showed supraventricular arrhythmia (two cats with supraventricular tachycardia, one cat from ATE group and another cat from CHF group; one cat with left anterior fascicular block from CHF group). Only one cat had a ventricular premature complex. Radiographical LA enlargement was detected in seven cats. For diagnosis of hypertrophy, end-diastolic wall thicknesses of the IVS and LVFW were measured. In both clinical groups, end-diastolic wall thickness exceeded 6.0 mm; however, there were no significant differences between cats in the two clinical groups. Routine echocardiographic examination also found no significant differences except for LAD. Cats in the ATE group had a significantly larger left atrium (P=0.026) than those in the CHF group. In both the ATE and CHF groups, LA:AO ratio increased (>1.6). However, we did not detect any significant differences between the two clinical groups with regard to LA:AO ratio, presence or absence of SAM, and thrombi using 2D measurements (Table 4).
ATE; arterial thromboembolism, CHF; congestive heart failure, LVIDs/LVIDd; left ventricular internal dimension at end-systole/end-diastole, LVFWs/LVFSd; left ventricular free wall thickness at end-systole/end-diastole, IVSs/IVSd; interventricular septum thickness at end-systole/end-diastole, FS; fractional shortening, LA/AO; left atrial to aortic root ratio, LAD; diameter of left atrium, SAM; systolic anterior motion.
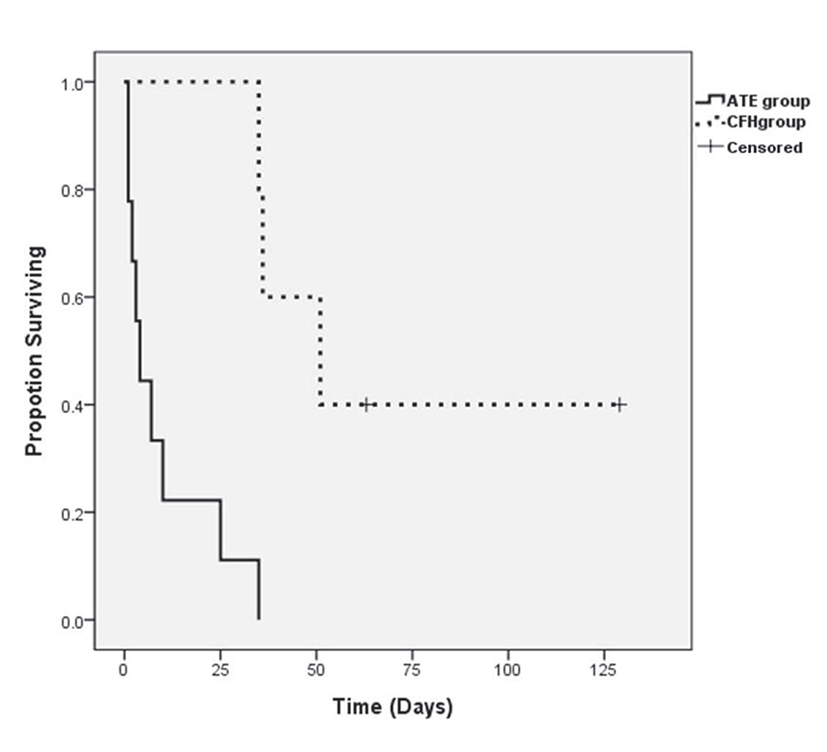
During the follow-up period, seven cats died due to cardiogenic causes (five cats from ATE group and two cats from CHF group), four cats were euthanatized due to deteriorated clinical signs (three cats from ATE group and one cat from CHF group), and two cats (CHF group) were still alive at the time of the study. The median survival times were 5.5 days (1 to 35 days) in the ATE group and 51 days (36 to 129 days) in the CHF group, and these values were significantly different (P=0.001; Fig. 1).
Discussion
HCM is the most common form of heart disease in cats, and several previous studies have evaluated retrospective characteristics and prognostic factors of HCM [1, 6, 8]. Certain breeds (Ragdoll and Maine Coon) [4, 6], presence of clinical signs at diagnosis [1, 5, 8], increased LA size [5, 9, 12], and old age [1, 4, 5] are considered as negative prognostic factors of HCM.
The cats in the present study were middle-aged (6 years), which is similar to other previous reports (5.9~7.0 years) [1, 6, 8]. Castrated male cats constituted slightly over half of the study population, and most cats included in this study were domestic short hair breed. Cardiac murmur was detected in only 33.3% of symptomatic HCM cats, which is similar to previous studies. Absence of murmur at diagnosis is negatively associated with HCM prognosis [1, 9], and systolic cardiac murmur is more common in asymptomatic than symptomatic HCM cats [4, 6].
In this study, we mainly focused on symptomatic HCM cats brought to emergency service. Based on this, the study population was divided according to clinical signs at presentation (ATE vs CHF group). Cats presenting with clinical signs of ATE constituted 61.5% of this study population while cats showing CHF signs without ATE constituted 38.5%. The most common initial clinical signs were pelvic limb paralysis (61.5%), depression (53.8%), and respiratory distress (38.5%). This result differs from previous reports. HCM is a well known underlying disease of ATE in cats [13, 14], and hypercoagulability status was previously found in 45% of cats with HCM [15]. However, most previous studies reported variable percentages of asymptomatic cats within the HCM population (33.5~77.3%) [1, 4, 5, 6]. One study measured HCM in 15% of apparently healthy cats [7]. Thus, ATE cats comprised 6~16.5% of their study population while CHF cats comprised 31.2~46.2% [1, 4, 5]. Due to differences in clinical status at diagnosis, our study showed higher prevalence of ATE in symptomatic HCM cats. Cats in the ATE group were slightly younger (median 5 years) than those in the CHF group (median 8 years); however, there were no clinical differences between the two groups.
End-diastolic IVS or LVFW hypertrophy (>6 mm) was observed in both the ATE and CHF groups; however, the result was not statistically significant. Thickness of LV hypertrophy was shown to be negatively associated with survival time in some studies [9, 12], whereas no association was found in another study [5]. A more recent study detected an association between extreme hypertrophy (>9 mm) and increased risk of cardiac death [1]. In our study, the ATE group showed a larger left atrial size than the CHF group (P=0.026). Enlargement of LA size could be associated with ATE, as blood stasis in the LA leads to platelet activation and local thrombus formation [12]. Increased LA size was a commonly recognized a negative prognostic factor [5, 8, 12].
Most studies have reported that cats with ATE or CHF have the shortest survival times [1, 5]. In previous studies, median survival times of ATE and CHF cats were shown to vary from 61 to 184 days and from 92 to 563 days, respectively [1, 4, 5]. The median survival times of the overall population were longer and were shown to vary from 492 to 2153 days [1, 4, 5, 6, 8, 9]. In this study, cats with ATE had a mean survival time of 5.5 days (range 1 to 35 days) while cats with CHF had a mean survival time of 51 days (range 36 to 129 days). The median survival time of our study was comparatively shorter than those of previously published studies. As mentioned earlier, this study focused only on symptomatic HCM cats receiving emergency services. Thus, clinical status of cats in our study might be worse than those in other studies. Hematologic evaluation revealed that muscle-related enzyme and tissue necrotizing-related enzymes were extremely elevated in the ATE group. In addition, ATE was detected during management or follow-up periods in other studies, and our study included cats based on their initial clinical signs. We also evaluated time to hospital visitation after initial clinical signs were detected by the owner. Two days and 4.8 days were taken for initial treatment after clinical signs in the ATE and CHF groups, respectively. According to previous reports [13, 14, 17, 18], over 30% of cats with ATE were euthanized in the initial stage of disease based on guarded prognosis. For treatment of ATE, thrombolytic drugs should be used within 4 hours after appearance of clinical signs to maximize their effects [19]. Similar to previous reports, 30.8% of cats were euthanized in our study, and this also influenced the short survival time of our study population.
This study has several limitations. First, we tried to restrict or exclude cats with predisposing factors to HCM, concurrent disease, and asymptomatic cats. Due to the low number of cats in our study, we could not evaluate prognostic factors of increased risk of death. Breeds disposition could not be evaluated due to the small number of cases. We also found no statistical differences in several factors showing differences in previous reports. In addition, our study population was derived from emergency care centers. Thus, the present study population may have suffered from more critical clinical signs, which could have affected the clinical outcome of the study.
In conclusion, this report demonstrated that pelvic limb paralysis, depression, and respiratory distress were relatively common clinical signs in symptomatic HCM cats. Most of the cats were middle-aged, castrated male domestic short hair cats. In the ATE group, muscle damage and tissue necrosis-related enzymes were elevated, and the cats also showed a larger LA size than the CHF group. Due to the different study populations, clinical status of initial presentation, and selection of timing for euthanasia, the survival time of our study was shorter than those of previous reports. Prospective investigation based on a large population would be required to clarify the effects of various factors on prognosis of HCM cats in our country.







