Introduction
Bladder cancer (BC) is a heterogeneous disease, which means that pathologically similar tumors may behave differently. In approximately 70% of all BC cases, patients present with non-muscle invasive bladder cancer (NMIBC), whereas the remaining 30% present with muscle invasive bladder cancer (MIBC). The standard treatment for NMIBC is transurethral resection (TUR) complemented by intravesical immunotherapy or chemotherapy to prevent recurrence and progression [1,2]. Numerous factors are likely involved in disease outcome, and many patients with NMIBC experience disease recurrence and progression after primary treatment [1,2]. Therefore, identifying patients at high risk of recurrence and progression who would benefit from more aggressive treatment, as well as those at low risk who require less intensive surveillance after initial adequate therapy, is challenging. Currently, conventional clinicopathological factors are insufficient to predict the outcome of patients with NMIBC. Thus, additional biomarkers are needed for prognosis of NMIBC patients.
Recent advances in our understanding of epigenetic modifications, including DNA methylation, histone modifications, and microRNAs, have provided new opportunities for detecting, treating, and preventing cancer. The usage of DNA methylation as a biomarker has attracted attention in recent years since aberrant DNA methylation is a major characteristic of BC and plays a crucial role in tumor initiation and progression [3-6]. DNA methylation, which inactivates tumor suppressor genes, is the most common and well-characterized epigenetic change in human cancer and may be a potential biomarker for cancer [7,8]. Previous studies have found that GATA-binding protein 5 (GATA5) hypermethylation and associated epigenetic silencing may be involved in carcinogenesis of various tumors such as BC, renal cell carcinoma, and gastric, colorectal, and ovarian cancers and are related with tumor aggressiveness and patient prognosis [9-17].
To the best of our knowledge, relatively few studies have evaluated the association between GATA5 methylation status and BC [16,17]. The aim of the present study was to evaluate the effect of GATA5 methylation status on clinicopathological features and prognosis in primary NMIBC patients with a long-term follow-up period.
Materials and Methods
A total of 171 human bladder tissues (eight normal controls [NCs] and 163 NMIBCs) were used for pyrosequencing (PSQ) analyses (Table 1). Primary NMIBC tissues were obtained from patients who underwent TUR for histologically diagnosed transitional cell carcinomas between 1995 and 2012 at Chungbuk National University Hospital. To exclude the possibility of incomplete resection or factors that may affect analyses, patients who were followed for less than 6 months or those that experienced disease relapse within 6 months were excluded from this study. NC tissues were obtained from individuals with benign prostate hyperplasia or bladder injury.
All tumors were macro-dissected within 15 min of surgical resection. Each NMIBC specimen was confirmed by pathological analysis of a tissue section that was obtained from the TUR specimens, immediately frozen in liquid nitrogen, and stored at – 80°C. The specimens were provided by Chungbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare, and Family Affairs. Collection and analysis of all samples were approved by the Chungbuk National University Hospital Institutional Review Board (GR2010-12-010), and informed consent was obtained from each subject.
Tumor staging was classified according to the 2002 TNM classification and 1973 World Health Organization grading systems [18]. Patients with intermediate- or high-risk NMIBC received one cycle of intravesical instillation therapy. Each patient was followed up and managed according to standard recommendations [1,2]. Recurrence was defined as the return of primary NMIBC at a lower or equivalent pathologic stage (Ta/T1), and progression was defined as muscular invasion (TNM stage T2 or higher) or nodal/distant metastatic disease.
Genomic DNA was extracted by standard methods using the Wizard Genomic DNA Purification System (Promega, Madison, WI, USA). Bisulfite conversion of genomic DNA was carried out using an EZ DNA Methylation Kit (Zymo Research, Irvine, CA, USA). DNA methylation status of GATA5 was assessed by PSQ using PyroMark Q96 ID (Qiagen, Valencia, CA, USA). Primer sequences were as follows: forward primer: TGTGGTAGTTGGTGTAGTAGAG, reverse primer: (Biotin) -AATCTCCCTCCCCCCCACAATC, sequencing primer: GTTGGTGTAGTAGAGG, sequence to analyze: TYGGYGYGGYGGGAYGAGGATTGTGGGGGT. The PCR reaction mixture contained 0.01 μM primers, Bioneer Taq (Bioneer, Daejeon, Korea), and 20 ng of bisulfite-treated DNA.
Thermocycling parameters were as follows: denaturation at 94°C for 5 min, followed by 45 cycles of 94°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 30 sec, and a final extension at 72°C for 5 min. A biotin-labeled primer was used to purify the final PCR product using streptavidin-coated Sepharose beads (GE Healthcare, Wauwatosa, Wisconsin, USA). The PCR product was bound to Sepharose beads, purified, washed, denatured using 0.2 M NaOH solution, and washed again. Subsequently, 0.3 μM PSQ sequencing primer was annealed to the purified single-stranded PCR product, and PSQ was performed on a PyroMark Q96 ID (Qiagen, Valencia, CA, USA). Target CpG sites were evaluated using the instrument software (PSQ96MA 2.1, Qiagen, Valencia, CA, USA), which converts pyrograms to numerical values for peak heights and calculates the proportion of methylation at each base as a C/T ratio. Data analysis was performed using PyroMark Q96 ID Software v.1.0 software (Qiagen, Valencia, CA, USA).
Differences in GATA5 methylation values between groups were assessed using a two-sample t-test or ANOVA trend analyses using polynomial contrasts. Median values were used as a cut-off point to divide patients into subgroups (hypomethylation or hypermethylation), and survival functions of GATA5 genes were evaluated. The Kaplan-Meier curves were used to estimate time to recurrence or progression according to methylation status, and differences were evaluated using log-rank tests. Using multivariate Cox proportional hazards regression analyses, the prognostic value (recurrence or progression) of methylation status was evaluated and adjusted for well-known clinicopathological factors (sex, age, tumor size, tumor number, intravesical therapy, grade, and stage). Statistical analysis was performed using SPSS 20.0 software (IBM, Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
Results
Baseline characteristics of NC and NMIBC patients are presented in Table 1. Mean age was 63.2 ± 13.8 years for patients with NMIBC. Mean recurrence- and progression-free survival times were 47.3 ± 40.1 months (median, 35.9; range, 6.1 to 171.5) and 62.3 ± 41.7 months (median, 52.3; range, 6.6 to 171.5), respectively.
As shown in Table 2, methylation levels of GATA5 were significantly higher in samples from NMIBC patients (53.6 ± 24.7%) than in those from NC patients (17.6 ± 3.6%) (p<0.001). To evaluate the relationship between methylation patterns and clinicopathological factors, methylation levels were compared with well-known prognostic factors such as tumor number, size, grade, and stage. High levels of GATA5 methylation were significantly associated with higher grade and more advanced stage tumors (each p<0.001).
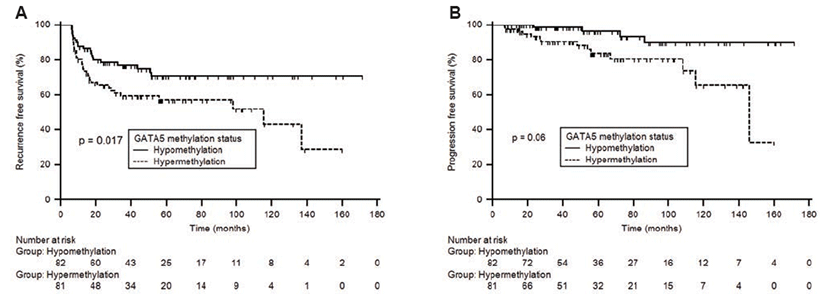
To evaluate the effect of GATA5 status on prognosis (recurrence or progression), we compared GATA5 methylation level based on prognosis. The GATA5 methylation level was significantly higher in the poor prognosis group (recurrence or progression) compared to the favorable prognosis group (Table 2). To further determine the correlation between methylation and prognosis, GATA5 methylation levels of each patient were dichotomized (hypomethylation or hypermethylation) with the median defined as the cut-off point. Kaplan-Meier estimates revealed that the GATA5 hypermethylation group had significantly less time to recurrence and progression than the GATA5 hypomethylation group (Fig. 1, log-rank test, each p<0.05). Univariate Cox regression analyses showed that GATA5 methylation status was a predictive factor of recurrence (hazard ratio [HR], 1.908; 95% confidence interval [CI], 1.110~3.279; p=0.019) and progression (HR, 3.725; 95% CI, 1.213~11.439; p=0.022) in patients with primary NMIBC. Upon multivariate Cox regression analyses, GATA5 methylation status served as an independent predictive factor of recurrence (HR, 1.470; 95% CI, 0.818~2.643; p=0.128) and progression (HR, 2.554; 95% CI, 0.758~8.603; p=0.130) in patients with primary NMIBC, although it did not reach statistical significance.
Discussion
Our results show that methylation level of GATA5 was significantly higher in tissues from NMIBC patients compared to NC patients, and hypermethylation of GATA5 was significantly associated with higher tumor grade and advanced pathological stage. Although GATA5 methylation status was not an independent prognostic indicator of recurrence and progression, it was significantly associated with reduced time to recurrence and progression in primary NMIBC patients.
Genetics refers to the study of information inherited on the basis of gene sequences, whereas epigenetics is the study of reversible and inheritable changes in gene function, or of other cell phenotypes without alteration of DNA sequences. DNA methylation occurs throughout the genome and involves the addition of a methyl group to the cytosine ring of the CpG dinucleotide [3]. The methylation pattern is established during development and is normally maintained throughout the life of an individual. Thus, DNA methylation is a key regulator of gene transcription and genomic stability, and inappropriately altered DNA methylation patterns are frequently detected as epigenetic changes in human cancers. In BC, hypermethylation of tumor suppressor genes such as APC, ARF, CDH1, GSTP1, MGMT, CDKN2A, RARβ2, RASSF1A, TIMP3, and RUNX3 has been reported [4-6]. As promoter hypermethylation is frequently observed in BC, several authors have investigated its occurrence in exfoliated urinary cells or tumor tissues. Methylation of these genes may facilitate cancer detection and correlate with a poor prognosis [4-6]. Using survival as the end point, different studies have demonstrated that methylation of CDH1, FHIT, LAMC2, RASSF1A, TIMP3, SFRP1, SOX9, PMF1, RUNX3, and SYNPO2 is associated with poor survival in patients with MIBC [6]. Thus, markers for aberrant methylation may be a potential gateway for monitoring and determining prognosis of BC. In the present study, GATA5 hypermethylation was associated with aggressive tumor features and poor prognosis. Although GATA5 methylation status was not an independent prognostic indicator in multivariate analysis, the results suggest the possibility of GATA5 gene as a methylation-based biomarker in BC.
GATA transcription factors containing two conserved zinc finger DNA-binding domains recognizing the WGATAR sequence (W=A or T and R=A or G) play a critical role in regulating embryonic morphogenesis and cellular differentiation [19]. There exist six GATA factors divided into two groups, GATA1/2/3 and GATA4/5/6, according to tissue-specific expression pattern. GATA1, GATA2, and GATA3 of the GATA transcription factor family are involved in cellular lineage and hematopoietic development, whereas GATA4, GATA5, and GATA6 are involved in epithelial and endodermal differentiation [20,21]. GATA6 may function as an oncogene since it is often up-regulated in proliferating progenitor cells [22]. In contrast, GATA4 and GATA5 may function as tumor suppressors since they are potential up-regulators of differentiation-related genes in endoderm-derived organs [23]. In addition, allelic imbalances in chromosomal loci for GATA4 8p23.1-p22 and GATA5 20q13.2-q13.3 are frequent areas of chromosomal deletion in neoplasms [24]. Epigenetic alteration of GATA5 has been detected in various types of cancers and is closely related with tumor characteristics [9-15]. Recently, Peters et al. reported an association between GATA5 hypermethylation as well as metastasis and progression-free survival in patients with renal cell carcinoma [11]. Only a few reports have detailed the association between GATA5 methylation and BC [16,17]. A previous study on the methylation status of tumor suppressor genes and its utility for predicting BCG responses in 91 patients with T1G3 high-risk BC reported GATA5 gene as a novel methylation marker in BC [16]. Moreover, in their study, recurrence and progression rate were significantly associated with GATA5 methylation status. In another study, methylation status of GATA5 was used to classify pTa versus pT1 tumors as well as distinguish low grade versus high grade tumors [17]. In accordance with these previous studies, our results show that GATA5 methylation status was associated with aggressive pathological features (stage and grade) and poor prognosis (recurrence and prognosis) in patients with NMIBC [16,17].
A precise causal relationship between GATA5 hypermethylation and NMIBC has not yet been established. Additionally, even though GATA5 hypermethylation may have functional significance in BC, it may not play a crucial role in bladder tumor initiation or progression. Although we could not demonstrate the association between GATA5 hypermethylation and BC, it will be investigated in a further study. The objective of the present study was to identify methylation markers related to NMIBC. Thus, we focused on the association between specific methylation markers and disease phenotype rather than the effects of methylation status on gene transcription and function. The strength of our study was that we performed definitive subgroup analysis after selecting only primary NMIBC patients. As BC is a heterogeneous disease with a prognosis affected by many factors, it is important to evaluate useful genes as prognostic markers within a homogenous study population. Although GATA5 hypermethylation was not an independent predictor of disease outcome, the results presented herein are promising since clinical significance was evaluated in a relatively large number of human tissue samples obtained from primary NMIBC patients with a long-term follow-up period (median, 52.3; range, 6.6 to 171.5). Therefore, GATA5 hypermethylation might be serve as a biomarker for tumor prognosis, although further prospective and functional investigations will be necessary to reduce false prediction rates and ensure reliability. Therefore, not only large-scale validation studies of human samples but also functional analysis and gene ontologic evaluation of GATA5 genes should be performed to gain more insights into their biological mechanisms and clinical relevance.
Our results show that increased methylation of GATA5 was significantly associated with not only aggressive characteristics but also poor prognosis in patients with primary NMIBC. Alteration of GATA5 methylation might be used as a biomarker for prediction of prognosis in patients with NMIBC. However, prospective and functional investigations are necessary to clarify the role of GATA5 methylation in future clinical management of patients with NMIBC.







