Introduction
Ex vivo culture of corneas, first used in the 1980s for corneal transplantation, is a method of keeping the cornea alive under culture conditions. This culture method is used to preserve the human cornea up to the time of transplantation [1] as well as to study the corneal response to drugs, various injuries, and transplants in animal eyes. Moreover, this method facilitates preclinical studies preceding animal studies in order to avoid unnecessary animal sacrifice [2, 3]. In corneal ex vivo culture, bovine, porcine, and rabbit corneas are most commonly used. Several studies have used human corneas in ex vivo culture; however, these are difficult to obtain. In comparison, bovine and porcine corneas can be easily obtained. The biology of the porcine eye is similar to that of the human eye and is therefore commonly used in ex vivo culture [4]. Whereas porcine corneal epithelium is slightly thicker, Bowman’s layer and the stroma are similar to those in the human eye [5].
In nature, the cornea is provided with nutrients and growth factors primarily from the aqueous humor. However, in ex vivo culture, an additional nutritional supply is needed, and culture media are used to maintain the excised cornea. In humans, Optisol®, which contains tissue culture medium 199, Earle’s balanced salt solution, minimum essential medium, HEPES buffer, chondroitin sulfate, dextran, vitamins, precursors of adenosine triphosphate, gentamycin, and streptomycin, is widely used to preserve donor corneas, which can be stored in medium for up to 14 days [6]. However, Optisol® is expensive, and the required storage temperature for corneas is 4°C [7]. Therefore, long-term preservation is difficult due to low cultivation temperature [8]. Thus, commercial organ culture media are used for this type of ex vivo culture, and various media supplements and compositions have been studied [9, 10].
In this study, to mimic blinking action, an air-liquid system was used with a rocker. In previous studies, most corneal organ culture models used static culture methods [11, 12]. However, in an in vivo situation, corneas are kept intermittently moist by blinking motion and are not submerged. Further, Richard et al. reported that static, submerged organ cultured corneas present epithelial, stromal, and endothelial edemas [13].
In the present study, we evaluated normal changes during short-term porcine corneal ex vivo culture with optical coherence tomography (OCT), low vacuum scanning electron microscopy (LV-SEM), and histological analysis.
Materials and Methods
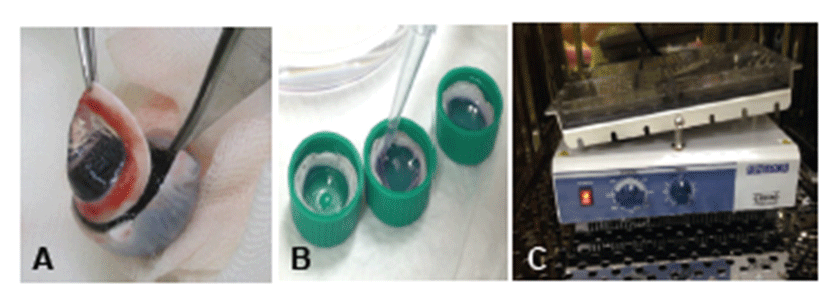
Eight pig heads were obtained from a local slaughterhouse (Samho Livestock Co., Gwangju, Korea), and eyes were enucleated within 4 hr of death. Enucleated eyes were soaked for 2 min in 0.2% povidone iodine solution for disinfection and were irrigated with sterile saline. For ex vivo culture, corneas were excised from the eye globe, leaving a 3~5 mm circular rim of sclera (Fig. 1A). After excision, corneas were filled with agar mixed with culture medium and transferred into culture dishes. Subsequently, culture medium was added until it covered the limbus (Fig. 1B). Culture medium consisted of Dulbecco’s modified Eagle’s medium (Gibco, USA)/F12 (Gibco, USA) supplemented with an insulin-transferrin-selenium mixture (BD Biosciences, USA), penicillin/streptomycin, and amphotericin B. Cultures were placed on a plate rocker and incubated at 37°C and 5% CO2 (Fig. 1C). Corneas were harvested on Days 0, 3, and 7 days after incubation commencement.
A custom OCT system was used in this study. A swept-wavelength light source (SSOCT-1310, AXSUN Technologies) was used with a center wavelength of 1310 nm, bandwidth of 107 nm, sweeping speed of 50 kHz, and imaging depth range of 6 mm in air. Light from the source was split with 95:5 ratios: 95% of light went into the interferometry setup, and the remaining 5% was directed to a fiber Bragg grating to generate an external trigger signal for data acquisition. For interferometry, light was split into the sample and reference arms by using an 80:20 coupler. In the sample arm, light was collimated to 1.8 mm in diameter with a fiber collimator (HPUCO-13A-1300/1550-S-11AS, OZ optics), passed through a 2D galvano mirror scanner (GVS012, Thorlabs) and an objective lens (LSM03, Thorlabs), and then focused at the sample. Light in the reference arm passed through a delay line in order to match optical path lengths in both arms. Reflected light from both the sample and reference arms was combined and collected by two balanced photodetectors (PDB410C, Thorlabs) in the polarization diverse detection setup. Signals from detectors were digitized by a two-channel data acquisition board (Alazartech) after passing through low-pass filters (81 MHz cut-off frequency, Mini-circuits). Stored data were processed to obtain OCT images. Dispersion difference between the reference and sample arms was compensated numerically by using precalibration data with a mirror sample. OCT images were obtained in 3D by acquiring multiple cross-sectional images in the x-z plane with step-wise increment in the y direction. Imaging field was typically 5 mm × 5 mm in the x and z directions, consisting of 500 pixels × 640 pixels. The signal-to-noise ratio of the system was 103 dB, and the frame rate was 100 frames/s. Central corneal thickness (CCT) was reported as the mean ± S.D. To compare OCT results between Days 0 and 7, an independent t-test was used. A P-value of <0.05 was considered to be statistically significant.
After OCT measurement, two corneas from each group were fixed in 2% glutaraldehyde/4% paraformaldehyde overnight at 4°C and dehydrated through an increasing series of ethanol solutions. Thereafter, specimens were submitted to critical point drying using liquid carbon dioxide and air-dried. Endothelial surfaces were photographed by LV-SEM (JEOL-JSM IT300, Tokyo, Japan).
After OCT measurement, four corneas from each group were fixed in 10% neutral-buffered formalin, dehydrated in an ascending series of alcohol rinses, and embedded in paraplast (Sherwood Medical Industries, USA). Embedded specimens were sectioned to a thickness of 5 μm with a microtome (Cambridge Instruments, Germany). Slides were stained with hematoxylin and eosin and evaluated for histological changes by microscopy.
Results
In OCT, mean CCTs on Days 0 and 7 were 868.53 ± 107.28 μm and 2925.00 ± 413.60 μm, respectively (Table 1). On Day 7, mean CCT had increased about 3.36-fold more than that on Day 0.
| Technique | Central corneal thickness (μm) | |
|---|---|---|
| Day 0 (n=6) | Day 7 (n=6) | |
| OCT | 868.53 ± 107.28 | 2925.00 ± 413.60* |
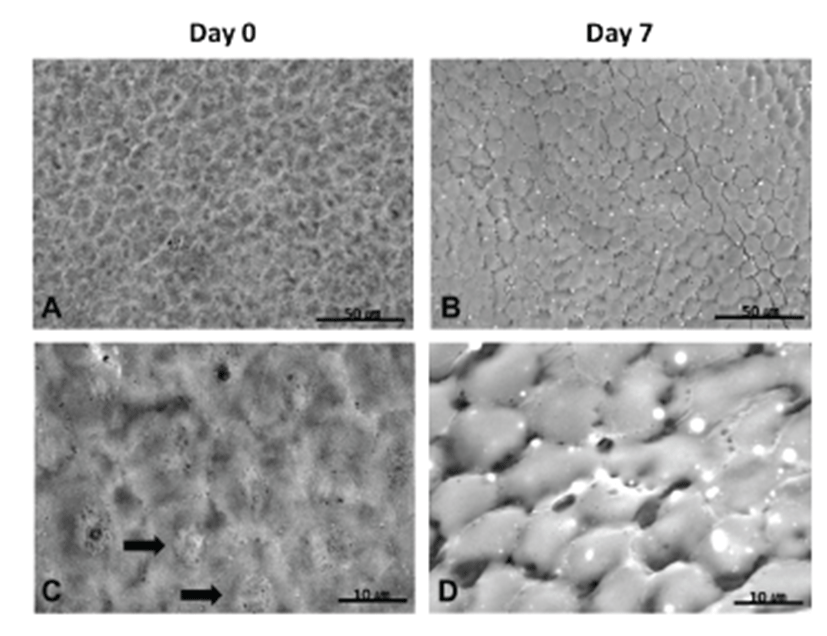
LV-SEM for corneal endothelium was performed on Days 0 and 7. On Day 0, a regular polygonal pattern was observed, and a round nucleus was detected in the center of the endothelial cell (Figs. 2A and 2C). On Day 7, a regular polygonal pattern of endothelial cells was also detected, although, gaps between endothelial cells in partial areas were found (Figs. 2B and 2D).
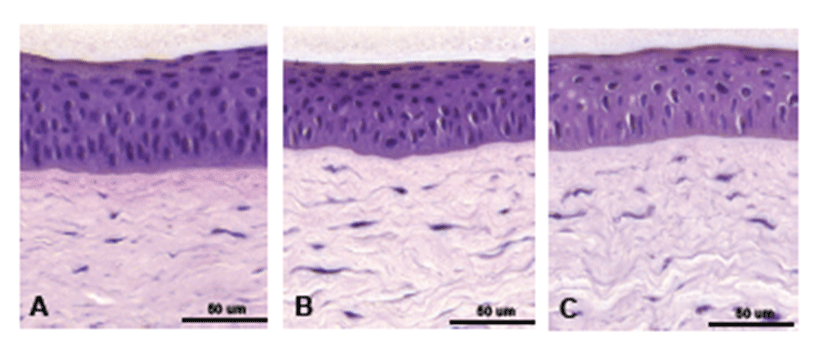
On Day 0, typically four to five layered, stratified squamous cells as well as wing cells and basal cells were found in the epithelium (Fig. 3A). On Day 3, overall epithelial thickness had decreased, and the stratified squamous cell layer and basement membrane were thinner than those on Day 3 (Fig. 3B). On Day 7, overall epithelial thickness was similar to that on Day 3. However, the stratified squamous cell layer decreased, along with numbers of wing cells and basal cells (Fig. 3C). In the stroma, parallel-arranged collagen lamellae and flat-shaped keratocytes were observed on Day 0 (Fig. 3A). On Day 7, slightly round-shaped keratocytes were found, although the overall population of keratocytes was not reduced (Fig. 3C).
Discussion
Ex vivo culture of intact corneas has many advantages over culture of small pieces of explanted corneas or corneal cells. First, cultured intact corneas are very similar to in vivo tissue [3]. Intact cornea has an intact epithelium, stroma, and endothelium, which maintains corneal hydration and cell signaling; the latter is very important for the wound healing process. Second, the limbus at the corneal margin has many stem cells and thus can provide a source of proliferative cells [14, 15]. Third, in wound studies, healing of wounded corneas in Ex vivo culture is a similar wound healing process as that in vivo.
Corneas can be cultured Ex vivo for up to 48 days [16]. However, corneal re-epithelialization after chemical burns or photorefractive keratectomy requires a shorter culture period (4~7 days) [17, 18].
To investigate normal changes in short-term corneal culture, Ex vivo culture was performed for 7 days with porcine eyes. Using OCT, we evaluated CCT and swelling of tissue. Using LV-SEM, corneal endothelium was evaluated for cell-cell interdigitation and morphology to observe the general status of the endothelium with histology. Using histological evaluation, corneal epithelium and stroma were identified. In OCT, the mean CCT on Day 7 increased by about 3.36-fold compared to Day 0. In LV-SEM, on Day 7, a regular polygonal pattern of endothelial cells was detected; however, several cells were separated at tight junctions. Additionally, in the histological findings, the stratified squamous cell layer was reduced, numbers of wing and basal cells had decreased in the epithelium, and slightly round-shaped keratocytes and stromal swelling were observed. However, the overall population of keratocytes had not decreased in the stroma on Day 7. These results suggest that corneal swelling was present on Day 7 possibly due to breaking-up of endothelial cell-cell junctions. The corneal endothelium forms a barrier between the aqueous humor and stroma in vivo. Tight junctions and metabolic-pump sites in the epithelium maintain corneal deturgescence, and destruction of the endothelium results in corneal edema and swelling [19]. Although the cause of corneal swelling in Ex vivo culture is unknown, one of the causative factors is endothelial-cell disruption, along with other factors such as metabolic changes in the culture medium, nutrient deprivation, mechanical stress, and loss of survival factors [20].
According to a similar study by Smith et al. [21], extensive wing cell death and epithelial detachment from the stroma occurred on Day 5 of ex vivo culture in Optisol®. However, there was no severe increase in corneal thickness. In contrast, epithelial thickness decreased, whereas epithelial detachment was not observed in any samples. Further, in histological evaluation, reduced keratocyte population was not detected. To confirm these results, further investigation such as keratocyte density assay is needed.
In conclusion, corneal ex vivo culture with organ culture medium is an effective culture method. However, to prevent corneal swelling, addition of growth factors or specified culture media that protect the endothelium or a deswelling agent should be considered in future studies.







