Introduction
Infective endocarditis (IE) is a rare disease affecting dogs and cats. A recent report showed its prevalence ranging from 0.04 to 0.13% [18]. Dogs suffering from IE are known to display non-specific and systemic clinical symptoms such as depression, weakness, lethargy, weight loss, anorexia, and fever. Even though endocarditis is a life-threatening disease, it has been neglected due to its difficulty of diagnosis. Several organisms have been reported as being associated with IE in dogs, mainly including Streptococcus, Staphylococcus, Escherichia coli, and Bartonella spp [19]. Chronic bacterial infections of the genitourinary tract, intervertebral disks, oral cavity, and skin may predispose dogs to occurrence of IE [19]. Infections with S. pseudintermedius are more common in dogs than in cats [7]. Transmission of S. pseudintermedius between humans and animals in a veterinary clinic has also been reported [7]. This implies that S. pseudintermedius can pose public health problems. In this case, multiplex-PCR and RFLP identified a causative agent and evaluated its several characteristics, including antimicrobial resistance and virulence factors.
Case report
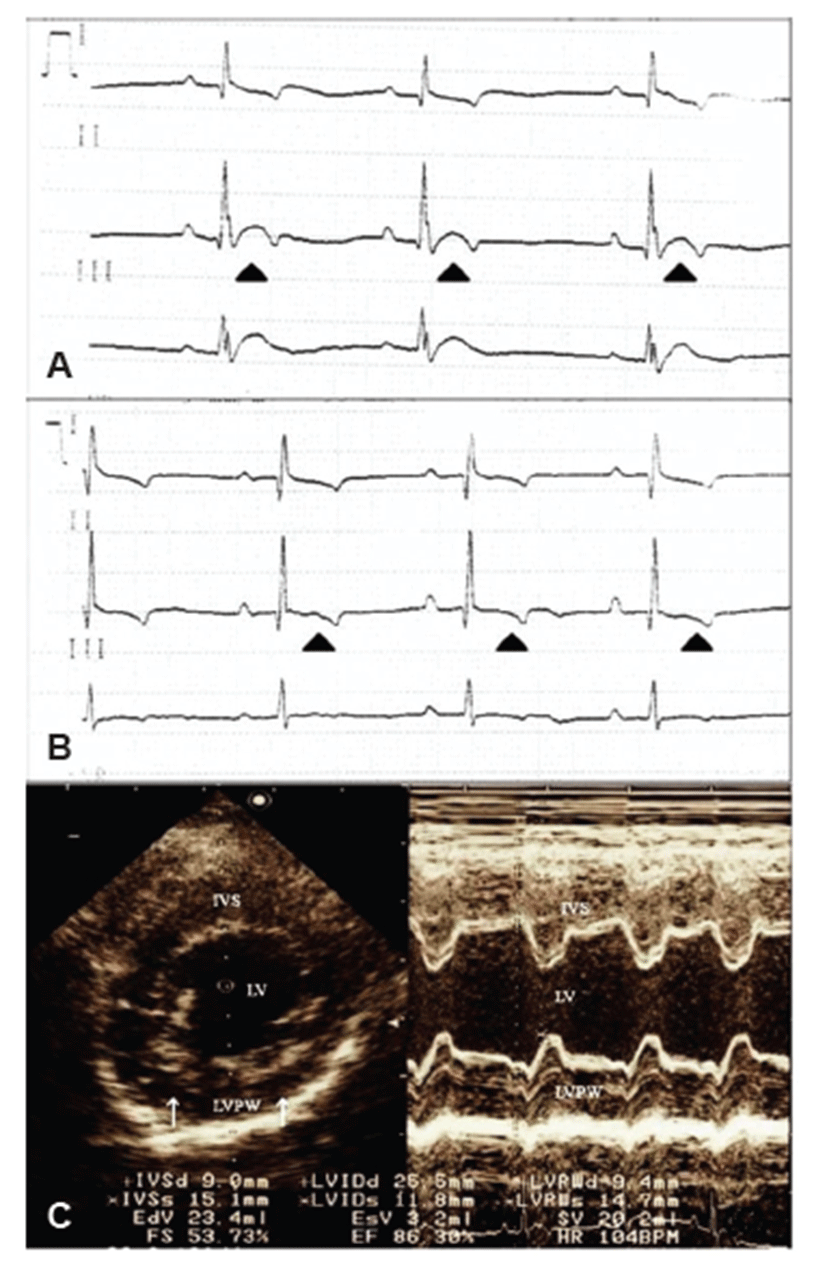
A 5-year-old, 8.95 kg, female Schnauzer dog presented acute anorexia with a 3-day history and weakness. Physical examination revealed depression, panting, and hyperthermia with a body temperature at 39.8°C. Markedly, increased heart sound intensity was auscultated. The complete blood counting results indicated leukocytosis (29,530/μL; reference, 4.9 to 15.4 × 103/μL) with neutrophilia (26,281/μL; reference, 2.9 to 10.6 × 103/μL). The serum chemistry profiles revealed hyper-cholesterolemia (370.64 mg/dL; reference, 135 to 345 mg/dL), hyper-glycemia (130 mg/dL; reference, 70 to 118 mg/dL), hyper-proteinemia (8.66 g/dL; reference, 5.4 to 7.4 g/dL), and hyper-albuminemia (4.29 g/dL; reference, 2.9 to 4.2 g/dL). However, thoracic and abdominal radiographs showed no remarkable changes. Electrocardiogram revealed a sinus rhythm with a rate of 90 bpm, notched R waves, and elevated ST segments, implying regional myocardial hypoxia and dysfunction (Fig. 1A). Echocardiography showed intact valves without regurgitation and some parts of the hyper-echogenic endocardium (Fig. 1C), which also indicated myocardial disorders and endocarditis. Abdominal ultrasonography showed bilateral, irregularly-shaped kidneys with both dilated medullar and increased cortical echogenecity of the right kidney. Hyper-echoic calculi with acoustic shadowing were also detected in the urinary bladder. Urinalysis revealed hematuria, proteinuria, pyuria, and ketonuria, although the specific gravity and pH were within normal ranges. Cytology of the urine showed numerous neutrophils (>5 white blood cells/ high-power field), bacterial cocci, and RBCs.
Based on these results, the dog was diagnosed with bacterial cystitis presenting calculi. Nonetheless, generalized weakness, increased cardiac intensity, and hyper-echoic endocardium could not be explained through the examinations since the dog had no other clinical signs of heart disease. Thus, the blood and urine were submitted for bacterial culture with aseptic procedures in order to rule out systemic infection-induced IE.
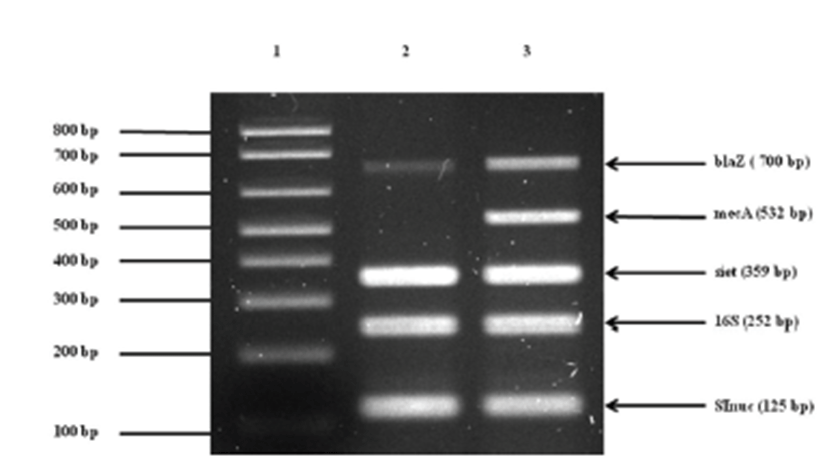
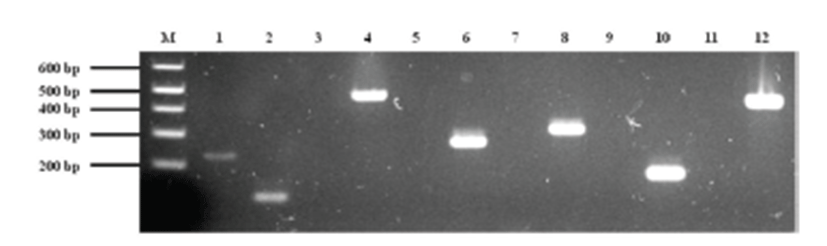
Treatment was initiated with empirical antibiotics for bacterial cystitis, including cephalexin (30 mg/kg p.o, q12h) and enrofloxacin (5mg/kg p.o, q12h), before the bacterial culture was obtained. The primary bacterial cultures from blood and urine (Green Cross Reference Lab, Gyeonggi-do, Korea) were positive for Staphylococcus spp. on the basis of colony morphology, complete or incomplete hemolysis, Gram-staining, and a conventional catalase test. To examine whether or not the isolate belongs to the S. intermedius group (SIG), multiplex-PCR was established and carried out using primers for S. intermedius nuclease (nuc), staphylococcal 16S rRNA, S. intermedius exfoliative toxin (siet), ß-lactamase (blaZ), and methicillin resistance (mecA) genes as previously described [1, 3, 22, 23]. For further differentiation of recently identified S. pseudintermedius from other SIG strains, a PCR-RFLP method was performed according to a recent description [2]. The results demonstrated that the canine isolates were S. pseudintermedius (Fig. 2). Additional toxotyping revealed that the S. pseudintermedius isolates carried no staphylococcal pyogenic toxin genes encoding staphylococcal enterotoxins A, B, C, D, and E or toxic shock syndrome toxin 1, except for SIET gene (Fig 3). All primers used in molecular diagnostics were as previously described [22, 23]. According to modified duke criteria [10], the dog was diagnosed with canine IE with S. pseudintermedius carrying SIET due to urinary tract infection.
After empirical antibiotics treatment for 3 days, the dog had no fever and showed typical heart sound intensity with normal ECG (Fig. 1B). These transient ECG changes may suggest diagnosis of infective endocarditis and sepsis-induced myocardial dysfunction [15]. While prolonged antibiotic therapy was carried out for another 7 days, clinical signs such as anorexia and depression were improved. The antibiotic susceptibility test of the bacterium showed that empirical antibiotics used for initial treatment were acceptable, so the antibiotics therapy was continued. One month later, the dog showed complete regression of clinical symptoms with normal status.
In this case, the dog did not show any murmur, and thoracic radiography and echocardiography did not show typical findings. According to a previous study [18], cardiac murmur was found in only 59% (41/71) of IE dogs. Actually, echocardiography could provide major evidence of diagnosis. However, only a few IE cases (2/57) showed echocardiographic abnormalities [18], which were confirmed at necropsy. Thus, vegetative pathologic changes in the valve may not be evident in the early stage of IE.
Discussion
Diagnosis of IE is challenging in particular due to a variety of non-specific clinical presentations, rapid disease progression, and lack of a confirmative diagnostic technique especially in its early stage. As it is quite difficult to diagnose IE, several investigators have proposed a series of diagnostic criteria [8, 13]. The modified duke criteria were used for diagnosis of IE in this case. According to the criteria, positive blood cultures with typical microorganisms are crucial for definitive diagnosis [10]. Even in the absence of positive blood culture, laboratory and physical examination findings consistent with systemic infection may be used to make tentative diagnosis of IE.
In general, blood culture has been used to identify the pathogenic agent of IE. However, existing microbiological tests have often produced false-negative results. Recently, new molecular tools have been suggested for diagnosis of IE due to an increase in negative blood-culture cases [6, 8, 17]. Furthermore, both antibiotic therapies and host immunity may also cause microbiological tests to fail despite use of appropriate laboratory techniques. Therefore, molecular detection and differentiation methods have been used as alternative diagnostic tools for IE. Indeed, molecular diagnostic tools have been applied for IE cases associated with certain fastidious, slow-growing, and/or non-culturable microorganisms [8]. Despite limitations in animal sample size, Sykes et al. tried to determine the relationships between clinical characteristics and infecting organisms among 71 clinical cases of canine IE [19]. In this study, the dog showed leukocytosis, hyperglycemia, hyperproteinemia, and hyperalbuminemia in the blood examinations. These blood examination results indicated early phase of systemic infection with dehydration status. According to a previous study, hyperglycemia is a common manifestation of the acute early host response [9]. Increased production of glucose in this situation transiently exceeds the rate of glucose disposal by peripheral tissues, resulting in the observed hyperglycemia [11].
The diagnosis of bacterial endocarditis in this dog was based on positive blood and urine culture results indicating Staphylococcus spp. The causative agent was then confirmed as S. pseudintermedius by PCR and PCR-RFLP. While determination of the causative agent was important for specific antibiotic treatment and prognosis, the results of ordinary bacterial culture and biochemical identification turned out to be incompatible with those of PCR and PCR-RFLP [5].
The recently identified S. pseudintermedius is a common microorganism that causes various skin-associated diseases in dogs and cats and has been occasionally isolated from serious human infections [12, 20]. Since the microorganism has zoonotic potential, the emergence and spread of methicillin-resistant S. pseudintermedius strains are a major concern in public health [14, 16]. Importantly, recent surveys suggested the high prevalence of methicillin-resistant S. pseudintermedius isolates with multiple drug resistance phenotypes [4, 7, 21, 22]. Although S. pseudintermedius infection has gained interest, there is still no report on identification of canine IE using molecular diagnostics.
In this report, additional evaluation of virulence factors was carried out to determine the relationship between a causative agent’s virulence factors and clinical signs. Staphylococcal pyogenic toxins such as staphylococcal enterotoxins A (SEA), B (SEB), C (SEC), D (SED), and E (SEE) and toxic shock syndrome toxin 1 (TSST) are members of the pyogenic toxin family. These toxins cause abnormal T lymphocytes activation and an impaired immune system. Although the pathological significance of SIET is not fully understood, a previous study showed that all S. pseudintermedius isolates from clinical cases of canine pyoderma in Korea carry the SIET gene [23]. It is assumed that SIET may promote bacterial invasion into the mammalian skin by cleaving certain intracellular adhesion molecules. In this case, the role of SIET was not exactly elucidated, but its cleaving function could assist invasion of the agent into the blood stream.
This case demonstrates the first isolation of S. pseudintermedius from canine IE using molecular diagnostics. Use of molecular technologies for isolation of bacteria has good accuracy. Additional information can be ascertained about the resistances of antibiotics and toxin typing, which makes it possible to diagnose IE earlier and predict a prognosis. Diagnosis methods with molecular technologies require further studies for establishment of guidelines. This method could replace previously IE diagnostic methods.







