Introduction
Heavy menstrual bleeding and dysmenorrhea affect daily life and health of women. Until the 1980s, hysterectomy was the standard treatment for heavy menstrual bleeding. Endometrial ablation was an alternative treatment for women who desired to save the uterus [1]; however, fertility could not be preserved in most cases. Women who desire to conceive are treated with progesterone, oral contraceptive pills, non-steroidal anti-inflammatory drugs, and tranexamic acid. However, except for continuous progesterone therapy, the efficacy of such treatments is limited. Oral contraceptive use is associated with a risk of venous thrombosis which increases with age [2, 3]. Therefore, it is not suitable for all middle-aged women. Although the levonorgestrel-releasing intrauterine system (LNG-IUS) was initially designed for contraceptive use, it is effective for treating heavy menstrual bleeding through the progestational effect on the endometrium, inhibition of endometrial growth, and induction of glandular atrophy [4]. Moreover, LNG-IUS has been used to treat endometrial hyperplasia and early endometrial carcinoma [5-7].
Ninety percent of patients with endometrial carcinoma have abnormal uterine bleeding and are diagnosed at early stages. Mostly, endometrial carcinoma occurs in postmenopausal women and 20% of the cases occur in premenopausal women. Routine endometrial sampling is mandatory before LNG-IUS use in all women.
This report describes a case of endometrial carcinoma that developed in an asymptomatic woman using LNG-IUS. The tumor was diagnosed at her routine health checkup and ascertained by elevated serum tumor markers.
Case report
A 48-year-old healthy, multiparous woman (non-smoker; body mass index, 22.5 kg/m2) who had been using the LNG-IUS for 3 years visited an outpatient clinic for a routine health check. Routine serum tests identified significantly elevated CA125 and CA19-9 levels (1,012.05 U/mL and 430.6 U/mL, respectively). The reference range of CA125 is less than 35 U/mL and that of CA19-9 is less than 37 U/mL. She was suspected to have pancreatic cancer and was referred to the Department of Gastroenterology at Chungbuk National University Hospital. The Torso fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) scan showed a large amount of FDG uptake (Fig. 1A) and the LNG-IUS (Fig. 1B) in the uterine cavity but no abnormality in any other organ. The patient was transferred to the Department of Obstetrics and Gynecology for further investigation.
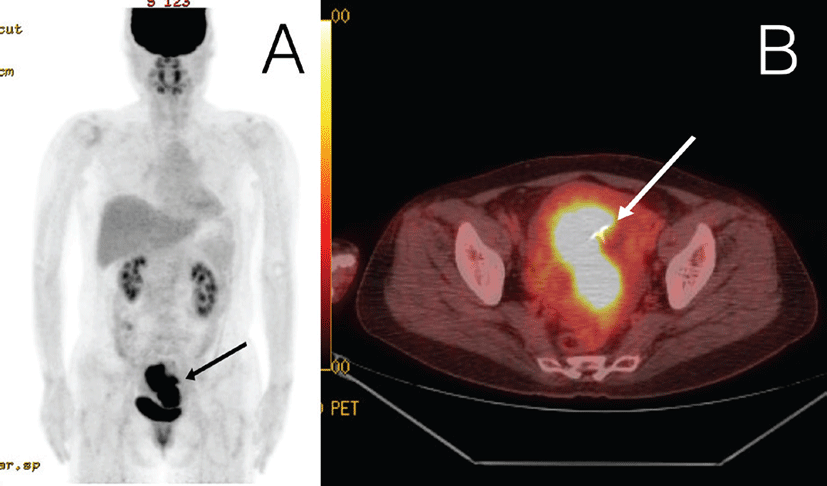
The patient’s history included heavy menstrual bleeding and severe dysmenorrhea for 5 years. Endometrial biopsy was performed at the time of her first visit, but had no abnormality. Further, LNG-IUS insertion was recommended for controlling bleeding and dysmenorrhea; however, the device was inserted 3 years later because of initial reluctance. During the procedure, endometrial thickness was 3~5 mm on ultrasonography. Following LNG-IUS insertion, the patient reported a vastly improved quality of life because the heavy bleeding and dysmenorrhea resolved. However, at a routine checkup after 3 years since LNG-IUS use, her serum CA125 and CA19-9 levels were elevated, although the levels at the 1-year and 2-year routine health checkup were within the normal range.
Pelvic ultrasonography showed a significantly thickened endometrium, the LNG-IUS, and a small amount of fluid present.
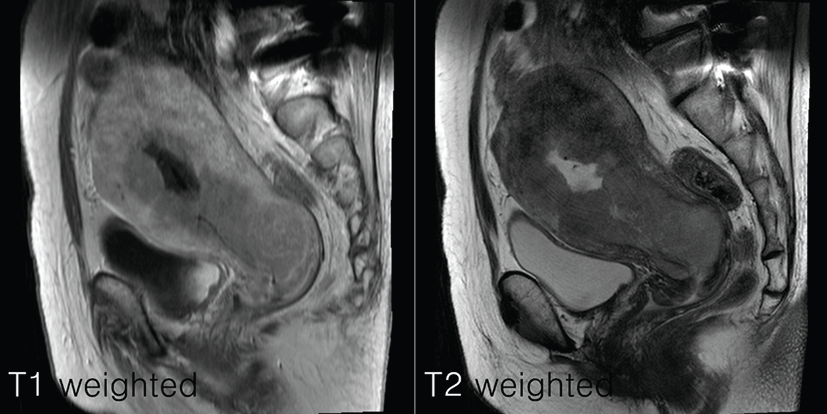
An endometrial biopsy identified poorly differentiated adenocarcinoma of the endometrioid type (Grade 3). Pelvic magnetic resonance imaging (MRI) indicated a mass with heterogeneous signal intensity typical of endometrial carcinoma. The mass appeared to have infiltrated more than half of the myometrium and the uterine cervix (Fig. 2) but not in the peritoneal surface; however, no abnormality was noted in the adnexa or pelvic lymph nodes.
The patient was subjected to radical hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy. Peritoneal washing cytological analysis of cells indicated no malignancy and histology revealed the same cell type throughout the tumor. The obturator lymph node was positive for malignant cells. Stage IIIC1 endometrial cancer was finally diagnosed based on International Federation of Gynecology and Obstetrics (FIGO) criteria.
On postoperative day 9, serum CA125 and CA19-9 levels had decreased to 75.1 U/mL and 69.8 U/mL, respectively. Concurrent chemotherapy and radiation therapy were given to reduce the risk of recurrence.
Discussion
LNG-IUS releases 20 μg levonorgestrel daily into the endometrial cavity and prevents intrauterine pregnancy by inhibiting endometrial proliferation and allowing decidualization [8]. It has been licensed as a contraceptive in the United Kingdom since 1995 and since 2004, its license has been extended for preventing idiopathic menorrhagia and endometrial hyperplasia in postmenopausal women receiving estrogen therapy [9].
Heavy menstrual bleeding in premenopausal women is the most common reason for hospital visits, owing to inconvenience and affects quality of life. The LNG-IUS is cost-effective for treating heavy menstrual bleeding and is a suitable alternative to surgical intervention [10-12]. The LNG-IUS has higher efficacy than hysterectomy in terms of increasing quality of life related to reduce menstrual flow or amenorrhea [13, 14].
Although the LNG-IUS prevents endometrial carcinoma by treating endometrial hyperplasia and early-stage carcinoma [5, 6], numerous endometrial carcinoma cases after LNG-IUS use have been reported recently. In 2002, Jones et al reported 2 cases: one woman had received cyclic combined estrogen and progesterone hormone replacement therapy (HRT) before LNG-IUS insertion; endometrial sample taken at that time was negative for carcinoma. The other woman reported had received the LNG-IUS without prior endometrial sampling. In the first case, it remains unclear whether HRT was implicated in the pathogenesis of the adenocarcinoma or whether the endometrial sampling was a false-negative result. In the second case, it remains unclear whether the patient already had an undiagnosed malignancy. Both women complained of abnormal uterine bleeding for several months before carcinoma diagnosis [15]. In 2006, Ndumbe and Husemeyer reported a patient who complained about abnormal uterine bleeding and weight loss but had not undergone endometrial sampling before receiving LNG-IUS. Therefore, it is unclear whether this patient had malignancy before receiving LNG-IUS [9]. Abu et al also reported a case of endometrial carcinoma in a young woman using the LNG-IUS for contraception who then complained of irregular bleeding [16]. In 2008, Flemming et al reported a case in which adenocarcinoma was detected on hysteroscopy despite a negative endometrial sample prior to insertion of the LNG-IUS. This patient had also experienced abnormal uterine bleeding for 8 months [17].
All patients with endometrial carcinoma identified after LNG-IUS insertion had symptoms such as abnormal uterine bleeding. However, after LNG-IUS insertion, characteristically, our patient was asymptomatic. The only indication of the disease was elevated serum tumor marker levels at her routine health checkup.
The combination of tumor marker levels and magnetic resonance imaging yields excellent positive and negative predictive values (90.9% and 88%, respectively) for endometrial carcinoma diagnosis [18-20]. Interestingly, our patient was initially referred to the Department of Gastroenterology due to elevated serum CA125 and CA19-9 levels and lack of other symptoms. Due to lack of a gynecological cause, pancreatic cancer was suspected. Immediately after screening for tumor marker levels, PET/CT was performed, the results of which indicated endometrial malignancy.
Neither PET/CT nor MRI showed lymph node metastasis, but pelvic lymphadenectomy revealed malignant cells in the obturator lymph node. Pelvic lymph node dissection should be mandatory for accurately classifying suspected advanced endometrial carcinoma, especially when more than half of the myometrium and/or uterine cervix has been infiltrated on imaging.
It is difficult to determine the point at which the carcinoma started to develop in our patient, but this development probably occurred <1 year before because serum tumor marker levels were within the normal range 1 year previously. The patient was satisfied with the outcome since LNG-IUS insertion. Although endometrial carcinoma was not suspected because of lack of abnormal uterine bleeding, it could have been detected earlier if regular follow-up, including measurement of endometrial thickness by ultrasonography, was performed at least every 6 months. Abnormal uterine bleeding was the common symptom in all women with endometrial carcinoma after LNG-IUS use. Thus, previous studies have emphasized the necessity of prior endometrial sampling, but no study has described the importance of regular follow-up. Although LNG-IUS is efficient and convenient for women with heavy menstrual bleeding, regular follow-up should be mandatory.
Our patient did not have endometrial carcinoma before LNG-IUS use, because endometrial sampling had shown negative results and endometrial thickness was measured as 3~5 mm on ultrasonography. The occurrence of endometrial carcinoma in this low-risk patient seems unconventional because she did not have any predisposing factors such as obesity or smoking; in addition, she did not take any estrogen compound. Although various factors may have contributed to the occurrence of this carcinoma, it remains unclear whether it occurred because of the LNG-IUS alone.
LNG-IUS insertion is an effective non-surgical method for treating heavy menstrual bleeding and severe dysmenorrhea. However, the likelihood of endometrial carcinoma, although rare, should be kept in mind; regular follow-up, including ultrasonography and measurement of serum tumor markers, is advisable, even in asymptomatic cases such as those without abnormal uterine bleeding. In addition to elevated tumor marker levels, PET/CT is useful for detecting malignant tumors when serum tumor marker levels are higher than normal. In cases in which imaging does not indicate lymph node metastasis, pelvic lymphadenectomy is necessary to detect deeply infiltrating endometrial carcinomas.







