Introduction
Probiotics have been defined as “live microbial feed supplement beneficial to the host (man or animal) by improving the microbial balance within its body” by Fuller [1]. In order to be used as beneficial probiotics it should have no pathogenicity and exhibition of specific properties such as gastric acid and bile tolerance, adhesion to intestinal epithelial cell surfaces, production of antimicrobial substances and immunomodulation effects [2].
Kimchi is well known as a traditional Korean fermented food. Kimchi fermentation is initiated by various endogenous microorganisms in the raw materials [3]. Many lactic acid bacteria (LAB) are involved in the fermentation of Kimchi such as Lactobacillus spp., Leuconostoc spp., Pediococcus spp. and Enterococcus spp. [3]. Enterococcus faecium JS1-8 strain was isolated from the Kimchi. Kimchi are known to possess several beneficial effects to health such as anti-diabetic effect, anti-oxidant action, anti-obesity effect, anti-cancer activity and immunomodulatory effects [4-8]. Although probiotic characteristics of Enterococcus faecium have been studied previously [9-11], there is little information on the immunomodulatory effects of Enterococcus faecium isolated from Kimchi.
Macrophages are phagocytes that play a central role in all phases of host defense including immune responses to infection. Macrophages can be activated by bacteria, lipopolysaccharides (LPS), and cytokines and activated macrophages regulate host immunity. Nitric oxide (NO) and pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are produced by macrophages [12]. NO is a short lived free radical that either kills or prevents the growth of bacteria and tumor. IL-1β is a member of the interleukin 1 family of cytokines. This cytokine is an important mediator of the proinflammatory response and is involved in a variety of cellular responses, including cell proliferation, differentiation, and apoptosis. TNF-α is mainly secreted by macrophages and cellular immunity activity and mediates resistance to infectious disease by controlling inflammation and pathogenic bacteria [13]. Nuclear factor κB (NF-κB) is a protein complex that controls transcription of DNA. It is involved in activation of pro-inflammatory genes. NF-κB plays a key role in regulating the immune response to infection by cytokine production [14].
The objective of this study was to examine the probiotic characteristics of Enterococcus faecium JS1-8 isolated from Kimchi and investigate the immuno-modulatory effects such as NO, NF-κB, IL-1β and TNF-α production in RAW 264.7 cells or RAW BLUE cells.
Materials and Methods
E. faecium JS1-8 isolated from Kimchi was used in this study and Lactobacillus rhamnosus GG (LGG, ATCC 53103), a well known commercial probiotic strain, was used as reference strains. As the indicator strain for antimicrobial activities and bacteriocin like substance producing activity, five types of microorganisms (Escherichia coli KCTC 1682, Salmonella enteritidis KCCM 12021, Staphylococcus aureus KCTC 1621, Listeria monocytogenes KCTC 3569, Lactobacillus sake KCCM 40264) were employed. JS1-8, LGG and L. sake were grown for 18 hr at 37°C in MRS broth (Difco, USA). The other pathogenic strains were cultured in BHI (Brain Heart Infusion) broth (Difco, USA) for 18 hr at 37°C before each experiment.
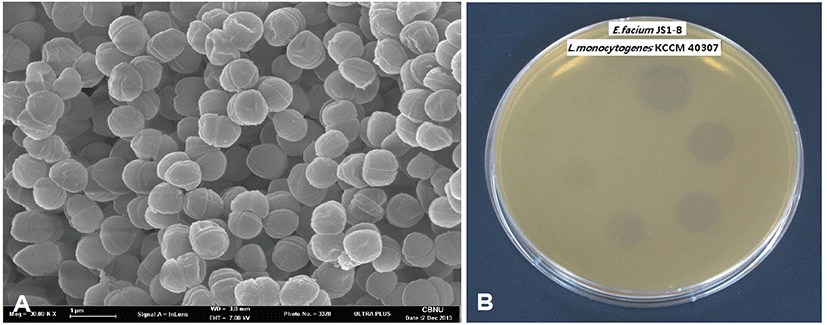
The monolayers of E. faecium JS1-8 were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 1 hr at room temperature. After washing with phosphate buffer, cells were fixed for 30 min with 2% OsO4 in the same buffer and washed five times. After dehydrated in a graded series of ethanol, cells were dried in a critical-point dryer (JEOL JCPD-5, Japan) and coated with gold. Specimens were observed by HITACHI S-4500 scanning electron microscope (Hitachi, Japan) [15].
In order to investigate the bacteriocin like substance producing activity, E. faecium JS1-8 strains were grown at 37°C in MRS broth for 30 hr. The bacterial cells were removed by centrifugation at 12,000 rpm for 15 min at 4°C and the supernatant was passed through a filter membrane (0.45 μm pore size) and then boiled for 10 min to inactivate the proteases. Ammonium sulfate was added to achieve 50% saturation. After precipitation for 24 hr at 4°C, the saturated solution was centrifuged 12,000 rpm for 20 min at 4°C. The pellet was resuspended in distilled water and dialyzed with a membrane with a 1 kDa cutoff (Spectrum Medical Inc., LA, USA) for 24 hr at 4°C. The antimicrobial activity of JS1-8 crude bacteriocin against several pathogenic bacteria and lactic acid bacteria (E. coli, KCTC 1682, S. enteritidis KCCM 12021, S. aureus KCTC 1621, L. monocytogenes KCTC 3569, L. sake KCCM 40264) were tested using spot-on-lawn method. Bacteriocin activity was expressed in terms of arbitrary units per ml (AU/mL) [16].
Acid and bile salt tolerance tests were conducted by a modified method of Kimoto et al [17]. To test tolerance at low pH value, JS1-8 strain suspended in MRS broth were adjusted with 0.05 M sodium phosphate buffer from pH 2.0 to pH 4.0 and incubated at 37°C for 2 hr. Bile salt tolerance was determined by cultivating on MRS plate containing 0.3% (w/v) oxgall (Difco Laboratories, USA). To assay the heat resistance, suspension of JS1-8 strain was aseptically inoculated into MRS broth and subjected to water bath at 70°C and 80°C for 5 min, respectively. After incubation for 48 hr at 37°C on MRS plate, viable cells were determined as acid, bile salt and heat tolerance [18].
The antagonistic activity of E. faecium JS1-8 strain was investigated by the modified spot on lawn method [19]. On top of each agar containing JS1-8, 7 mL of soft agar (0.7%, w/v) mixed with the indicator strain (107 CFU/mL, E. coli, KCTC 1682, S. enteritidis KCCM 12021, S. aureus KCTC 1621, L. monocytogenes KCTC 3569) was poured and followed by spotting 20 μL of concentrated culture supernatant upon each paper disc. The extent of the antimicrobial activity was measured by the diameter (mm) of the inhibition zone.
Human enterocyte-like Caco-2 cells were purchased from Korean Cell Line Bank (KCLB, Korea). Caco-2 cells were grown in DMEM (Gibco, USA), supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, USA) and 1% (v/v) streptomycin and penicillin (Gibco, USA. The number of adherent E. faecium JS1-8 per Caco-2 cell was determined by the average number of bacteria attached to the 10 Caco-2 cells. Adhesion property to Caco-2 cells was conducted according to Kimoto’s methods [17].
RAW 264.7 macrophages (5 × 105 cells/well) cells (KCLB) and RAW BLUE (5 × 105 cells/well) cells (InvivoGen) were cultured in DMEM supplemented with 10% FBS, 1% 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco). JS1-8 and LGG strain were killed by heating at 110°C for 15 min. All heat-killed probiotics were diluted to 5 × 108 CFU/mL for high concentration dosage or 1 × 108 CFU/mL for low concentration dosage. In contrast to probiotics, PBS was added as a negative control and LPS (100 or 1000 ng/mL; Sigma, USA) was added as a positive control in PBS containing wells. After 48 hr, the culture supernatants were collected for use in determining the productions of NO, NF-κB and cytokines (IL-1β and TNF-α) [12].
Nitric oxide productions were analyzed using Griess reagent (Promega, USA). Briefly, 50 μL of macrophage culture supernatant was mixed with 100 μL of Griess reagent and incubated at room temperature for 10 min in the dark. Samples were measured in triplicate. The absorbance was measured at 540 nm using a microplate reader (Tecan, Austria). NO levels in the supernatant were calculated using the generated standard curve of nitrite (0~100 μM sodium nitrite) [12].
Productions of cytokines (IL-1β and TNF-α) were measured by mouse ELISA kits (eBioscience, USA) according to the manufacturer’s instructions. Each assay was performed in triplicate with 450 nm in a microplate reader. Cytokine levels were then calculated with the standard curves of each cytokine (0~1,000 pg/mL for IL-1β and TNF-α) [12].
RAW BLUE cells were plated 5 × 105 cell/mL in each well of 12-well. Probiotics (100 μL volume containing 1 × 107 CFU/mL or 5 × 107 CFU/mL JS1-8 and LGG) were then added to the wells and incubated for 48 hr. As a positive control, 100 ng/mL lipopolysaccharide (LPS; Sigma, USA) was added. Medium samples (20 μL) were mixed with QUANTI-Blue (InvivoGen, San Diego, USA) medium (200 μL) in 96-well plates at 37°C for 15 min. The results of the secreted embryonic alkaline phosphatase (SEAP) activity were measured by reading absorbance at 620 nm using microplate reader (Tecan, Austria) [18].
Results
E. faecium JS1-8 strain was tested for their ability to survive under acidic conditions (pH 2.0) and for their bile tolerance (Table 1). JS1-8 could survive at pH 2.0 after 2 hr of exposure and also showed tolerance to 0.3% of oxgall bile salt. In the heat tolerance test, the JS1-8 was able to survive at 70°C and 80°C for 5 min, respectively. The average number of JS1-8 and LGG attached to the 10 Caco-2 cells were 81 and 100, respectively. JS1-8 strain showed adherence to the Caco-2 cells, but L. rhamnosus GG, a well-known commercial strain, had better adhesion capacity. Table 2 showed high antimicrobial inhibition zone of E. faecium JS1-8 strain to Staphylococcus aureus (460 mm), Listeria monocytogenes (310 mm), Salmonella enteritidis (280 mm), and E. coli (150 mm).
The antimicrobial activity of bacteriocin- like substance produced by E. faecium JS1-8 against several Gram-positive and Gram-negative bacteria was tested using modified spot-on-lawn method. The E. faecium JS1-8 showed a spectrum of antimicrobial activity against L. monocytogenes and L. sake. The greatest activity (3,200 AU/mL) was obtained from L. monocytogenes KCTC 3569. This bacteriocin-like substance did not show activity against Gram-negative bacteria such as Escherichia coli and Salmonella typhimurium (Table 2). Fig. 1.
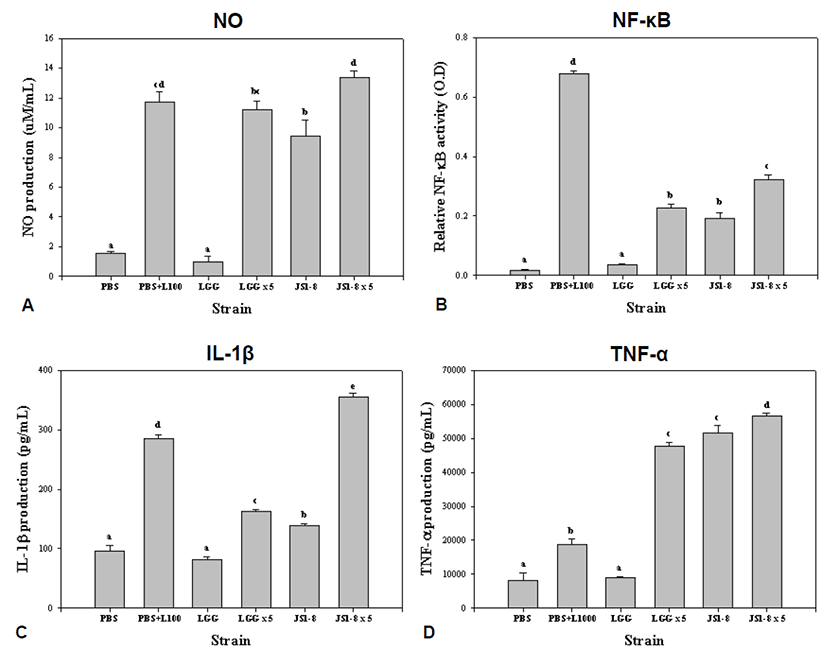
We measured NO, NF-κB and cytokines (IL-1β and TNF-α) induced by heat-killed E. faecium JS1-8 in activated RAW 264.7 or RAW BLUE cells to examine the immunomodulatory properties of JS1-8. Low concentration (1 × 107 CFU/mL) of JS1-8 induced statistically higher production of NO than LGG. High concentration (5 × 107 CFU/mL) of JS1-8 produced statistically higher production of NO than LGG, but these results were not statistically different from the 100 ng/mL LPS as a treated positive control (Fig. 2A). NF-κB production by E. faecium JS1-8 and LGG was increased in a dose-dependent manner. Low and high concentration of JS1-8 induced statistically higher production of NF-κB than that produced by LGG, respectively (Fig. 2B). Low concentration of LGG induced 82.17 ± 8.58 pg/mL of IL-1β production, which was significantly lower than that induced by E. faecium JS1-8 (138.17 ± 6.33 pg/mL). High concentration of JS1-8 (5 × 107 CFU/mL) induced 354.83 ± 10.87 pg/mL of IL-1β which is about 2-fold higher than that of LGG (162.75 ± 6.25 pg/mL) (Fig. 2C). Low concentration of JS1-8 induced significantly higher levels of TNF-α (51650 ± 3879.7 pg/mL) than the low concentration of LGG (8975 ± 375.0 pg/mL). High concentration of JS1-8 also produced statistically higher production of TNF-α than high concentration of LGG (47600 ± 1917.6 pg/mL) and 1000 ng/mL LPS (18800 ± 2560.7 pg/mL) (Fig. 2D).
Discussion
Potential probiotic strains should have tolerance for low pH/gastric juice and high pH/bile juice in order to successfully pass through the stomach and small intestine [20]. In the present study, E. faecium JS1-8 survived at pH 2 for 2 hr and showed resistance against 0.3 % of bile salt condition. This is in good agreement with the results of the study by Qianglai Tan et al [21], who found that E. faecium YF5 was tolerant to gastrointestinal transit in vitro.
Heat tolerance of probiotic bacteria is a desirable characteristic for stability during heat-processing. The heat tolerance of JS1-8 was tested in 70°C and 80°C for 5 minutes, respectively and survived well for an exposure of 5 min at 80°C. But Hosseini et al showed that all of the Enterococcus strains tested were killed after 5 min at 70°C and 80°C except only at 65°C [9]. As a result, although Enterococcus strains are a mesophilic bacteria, it seems to be flexible to heat treatment depending on the stains.
Adherent strains of probiotic bacteria are likely to persist longer in the intestinal tract and thus may have better chance to act through metabolism, antagonism against pathogens and modulation of the immune system than non-adherent strains [10]. E. faecium JS1-8 strain showed similar adhesion property compared with LGG, a well-known probiotic commercial strain.
In the present study, antibacterial activity was measured by the diameter of transparent inhibition zone against various food-borne pathogens such as Escherichia coli KCTC 1682, Salmonella enteritidis KCCM 12021, Staphylococcus aureus KCTC 1621 and Listeria monocytogenes KCTC 3569. These results revealed that the culture medium of E. faecium JS1-8 can effectively inhibit the tested pathogens. Previous study has demonstrated that probiotic strains usually have antibacterial effects because these strains produce several metabolic compounds such as organic acids, fatty acids, H2O2, or bacteriocins [22]. Du Toit M et al reported that E. faecium inhibited a wider range of indicator bacteria, including Listeria monocytogenes, Listeria innocua, Clostridium sporogenes, Clostridium tyrobutyricum and Propionibacterium spp [11].
In this study, only Gram-positive L. monocytogenes and L. sake were inhibited by the bacteriocin-like substances of E. faecium JS1-8. Generally, Gram-negative bacteria are not sensitive to bacteriocins produced by Gram- positive bacteria. Although the cytoplasmic membrane should be susceptible, the outer membrane protects the cell from bacteriocins. The outer membrane of Gram-negative bacteria acts as a permeability barrier for the cell [23]. But bacteriocin-like substances of E. faecium JS1-8 might be used to inhibit some pathogenic bacteria for probiotic use in humans and animals.
We measured the immune-modulating effects of E. faecium JS1-8 by analyzing NO, NF-κB and cytokines such as IL-1β and TNF-α compared with the LGG strain which is one of the best studied probiotic strains with its immune responses. We used heat-killed JS1-8 and LGG to prevent the cell death caused by overgrowth of probiotic cells and low acid conditions during experiment. As a result, the heat-killed E. faecium JS1-8 increased the production of NO and NF-κB. NO is a short lived free radical that either kills or prevents the growth of bacteria and it has been described to modulate intestinal barrier function, gut motility, iron transport, and be related to intestinal infection [24]. Activated NF-κB causes regulation of the genes involved in inflammation, cell proliferation and apoptosis. Also we found that E. faecium JS1-8 increased the production of pro-inflammatory cytokine such as IL-1β, and TNF-α in RAW 264.7 cells. These results show that E. faecium JS1-8 has stronger immunomodulating effects than LGG. Low (1 × 107 CFU/mL) and high (5 × 107 CFU/mL) concentration of JS1-8 induced statistically higher production of NF-κB, IL-1β and TNF-α than those produced by LGG, respectively.
In conclusion, E. faecium JSI-8 isolated from Kimchi showed excellent probiotic properties, wide spectrum of antimicrobial activity and enhancing host immune responses. E. faecium JSI-8 may potentially be used in feed and food industries as probiotic application to humans or animals and in natural biopreservatives. In addition, it could be a useful Kimchi starter and a health functional food for controlling food borne pathogens and modulating host immune responses.







