Introduction
The epidermis is a multi-layered epithelium, which krserves a role of physical barrier to protect the organism from the environment. It has been well known that much of this barrier function is provided by the cornified cell envelope (CE), a specialized insoluble structure formed beneath the plasma membrane during the process of keratinocyte differentiation [1-3]. Keratinocyte differentiation involves the process of cell cycle arrest and the onset of expression of numerous genes. For example, the expression of keratin 5 and keratin 14 is decreased, while the expression of several differentiation-related genes such as keratin 1, keratin 10, involucrin, loricrin and filaggrin is increased in a time-dependent manner [4, 5].
The importance of keratinocyte differentiation is supported by the fact that dysregulation of keratinocyte differentiation is directly linked to several skin diseases. For example, in psoriasis and atopic dermatitis, keratinocyte differentiation process incompletely occurs. As a result, skin barrier is disturbed and inflammatory reaction is exacerbated. The defect in skin barrier also results in excessive dry skin, which may be other exacerbating factor for differentiation-related skin diseases [6, 7]. Currently, immunomodulatory drugs such as cyclosporine A (CsA), tacrolimus and pimecrolimus are widely used as the first-line agents to treat differentiation-related skin diseases [8]. In addition, moisturization of skin gives beneficial effect on relieving disease status and enhancing skin texture [9]. In keratinocyte differentiation, final CE component filaggrin provides the natural moisturizing factor (NMF), thereby allowing the maintenance of healthy skin [10, 11]. Therefore, it is expected that therapeutics enhancing the keratinocyte differentiation may be used as the co-modalities together with first-line treatment.
Many investigators have concentrated their efforts on finding the effective materials for skin diseases. Medicinal plants are an important source of therapeutics and increasingly attractive all over the world. In this study, we prepared the extract of combined medicinal plants (ECMP) consisting of Taraxacum platycarpum H. Dahlstedt, Heartleaf Houttuynia, Glycyrrhiza uralensis Fischer and root bark of Ulmus davidiana. We demonstrated that ECMP enhanced keratinocyte differentiation and barrier functionality, suggesting that this material can be used for strengthening of skin texture.
Materials and Methods
Simian virus 40 large T antigen (SV40T)-transformed human epidermal keratinocytes (SV-HEKs) were maintained in keratinocyte-serum free medium (K-SFM) supplemented with bovine pituitary extract and recombinant human epidermal growth factor (Life Technologies Corporation, Grand Island, NY) [12].
All medicinal plants were obtained from Omniherb Co. (Daegu, Korea). Air-dried Taraxacum platycarpum H. Dahlstedt (80 g), Heartleaf Houttuynia (80 g), Glycyrrhiza uralensis Fischer (40 g) and root bark of Ulmus davidiana (40 g) were cut into pieces and extracted with 8 volumes of ethanol for 7 d. The extract was filtered using Whatman paper, evaporated under reduced pressure condition, and then freeze-dried (Eyela, Irvine, CA). For treatment of cultured cells, ECMP was reconstituted in dimethylsulfoxide (DMSO). For topical application, ECMP was reconstituted in 70% polyethylene glycol and 30% ethanol.
SV-HEKs (2 × 105) were seeded on 12-well culture plates then cultured for overnight. Next day, SV-HEKS were treated with ECMP at the indicated concentrations and incubated for 3 d. Cells received 2 mg/mL MTT solution and were incubated for further 4 hr. The medium was removed and the resulting formazan crystal was solubilized in 100 μL of DMSO. The optical density at 540 nm was determined using an ELISA reader.
Cells were lysed in Proprep solution (Intron, Daejeon, Korea). Total protein was measured using a BCA Protein Assay Reagent (Pierce Biotechnology, Rockford, IL). Samples were run on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes and incubated with appropriate antibodies. Blots were then incubated with peroxidase-conjugated secondary antibodies, visualized by enhanced chemiluminescence (Intron). The following primary antibodies were used in Western blot: involucrin (Cat# sc-21748), loricrin (Cat# sc-133757), filaggrin (Cat# sc-30229) (Santa Cruz Biotechnologies, Santa Cruz, CA), and actin (Cat# A2668) (Sigma, St. Louis, MO). Dilution factor for each antibody is 1:1,000.
For creation of involucrin-luc reporter adenovirus (Ad/Inv-luc) and loricrin-luc reporter adenovirus (Ad/Lor-luc), genomic DNA isolated from keratinocytes was used as a template for PCR. Primer sequences were as follows: involucrin promoter, 5’-CTCCATGTGTCATGGGATATG and 5’-TCAACCTGAAAGACAGAAGAG; loricrin promoter, 5’ TACCAAGCAATCCTCTCACCTTGG and 5’-TGAGGAGAGAAGATGCTGGC. The resultant PCR fragments cover from −2,467 to +1,239 base pairs of involucrin transcription site and from −2,033 to +12 base pairs of loricrin transcription site (http://www.ensembl.org/Multi/blastview). The promoter fragments were subcloned into pGL3 vector (Promega, Madison, WI), then moved to pENTR vector and adenoviruses were created.
SV-HEKs were grown at 50% confluency in 12-well culture plates and transduced with reporter adenovirus. After adenoviral transduction, cells were replenished with fresh medium and treated with ECMP. Cells were further incubated for 48 hr and then cellular extracts were prepared using cell lysis buffer. Luciferase activities were determined using Luciferase assay system (Promega, Madison, WI), according to the recommended protocol.
For [3H]thymidine uptake assay, SV-HEKs were seeded in 60-mm culture dish and treated with 1 μCi of [3H]thymidine (Amersham, Buckinghamshire, UK). Following incubation for the indicated time point, cells were washed twice with PBS and incubated with 0.1 N NaOH at room temperature. Radioactivity in cell lysates was measured by liquid scintillation counter.
Seven week old female hairless mice were purchased from Orient Bio Inc. (Seongnam, Korea). Skin barrier was disturbed by tape-stripping and then ECMP was topically applied. TEWL was measured using Tewameter TM210 (Courage + Khazaka electronic GmbH, Cologne, Germany) as previously described [13].
For skin sampling, mice were euthanized by cervical dislocation and then skin specimens were obtained by dissection with scissors. Skin samples were fixed in 10% formalin, and embedded in paraffin. Paraffin sections of skin specimens were de-waxed, re-hydrated and washed three times with PBS. Sections were then incubated with proteinase K (Dako, Carpinteria, CA) for 5 min at 37°C, treated with H2O2 for 10 min at room temperature, blocked in 0.1% Tween-20, 1% bovine serum albumin in PBS for 30 min, and followed by reaction with anti-mouse filaggrin antibody (Cat# ab24584, Abcam, Cambridge, MA) for 1 hr. Sections were incubated sequentially with peroxidase-conjugated secondary antibody and visualized with Chemmate envision detection kit (Dako, Carpinteria, CA).
Results
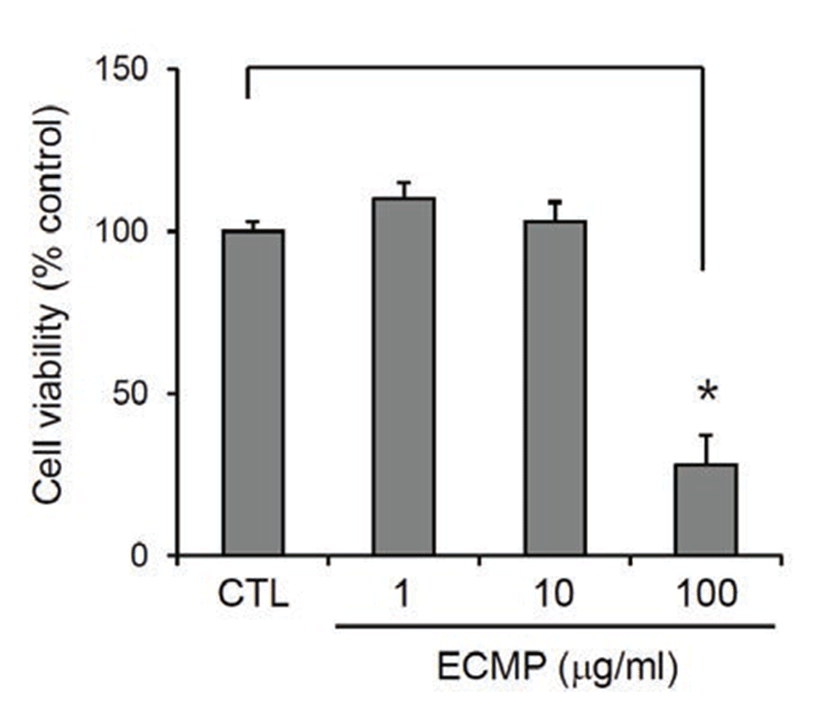
We prepared ECMP using 4 medicinal plants that are used frequently in oriental medicine. First, we determined the cytotoxicity of ECMP on SV40T-transformed human epidermal keratinocytes (SV-HEKs). After treatment for 3 d, ECMP showed no cytotoxicity up to the dose of 10 μg/mL (Fig. 1).
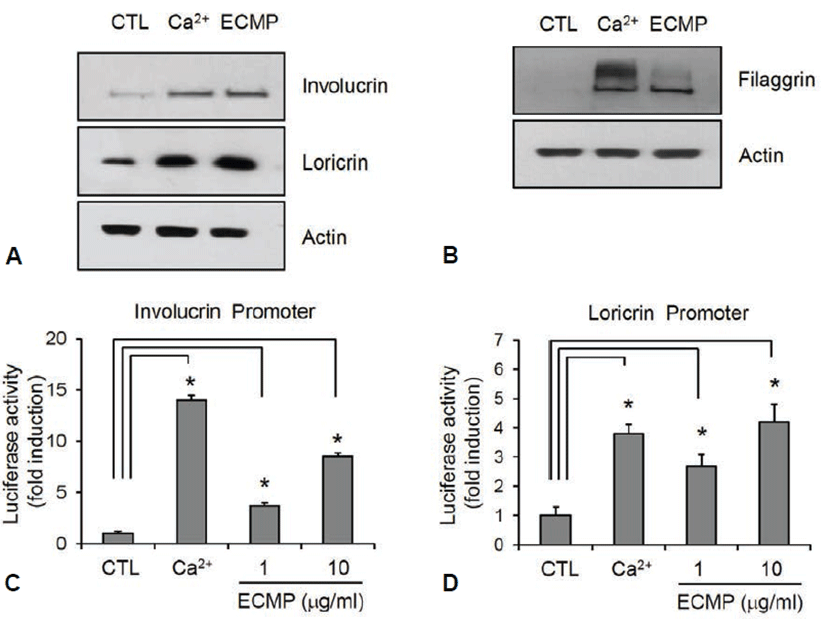
Next, we investigated the effect of ECMP on keratinocyte differentiation. We used calcium, a best known differentiation inducer [14], as a positive control. SV-HEKs were treated with ECMP for 3 d and the data showed that protein level for involucrin and loricrin was markedly increased by ECMP (Fig. 2A). Late differentiation marker filaggrin was also significantly increased after 7 d treatment with ECMP (Fig. 2B). To investigate whether ECMP affected the involucrin and loricrin expression at the promoter level, we tested the promoter activity using the recombinant adenoviruses harboring reporter constructs. As a result, ECMP induced involucrin and loricrin promoter activity (Figs. 2C and 2D). These results indicate that ECMP has a potential to enhance keratinocyte differentiation.
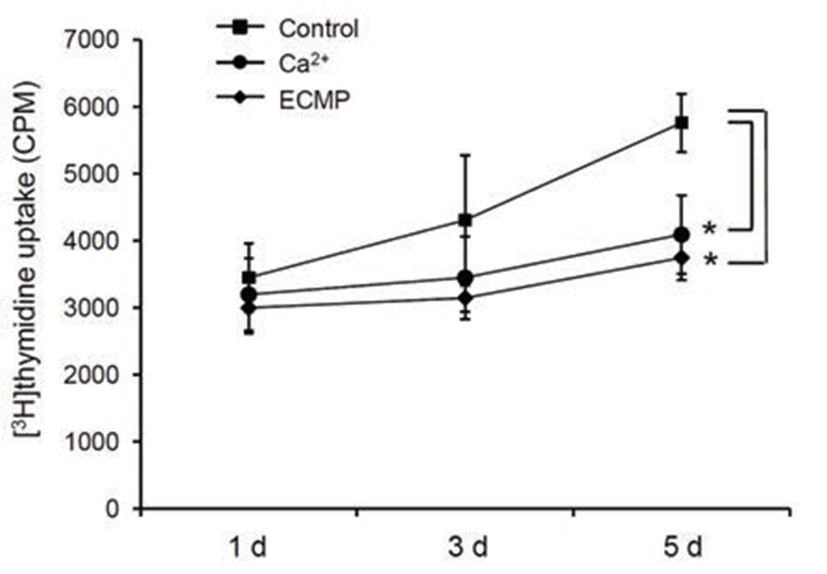
Since keratinocyte differentiation takes place along a pathway that leads to cell cycle arrest simultaneously, we next investigated the effect of ECMP on cell growth using [3H]thymidine uptake assay. ECMP treatment resulted in retardation of cell growth, similar to the calcium-treated group (Fig. 3).
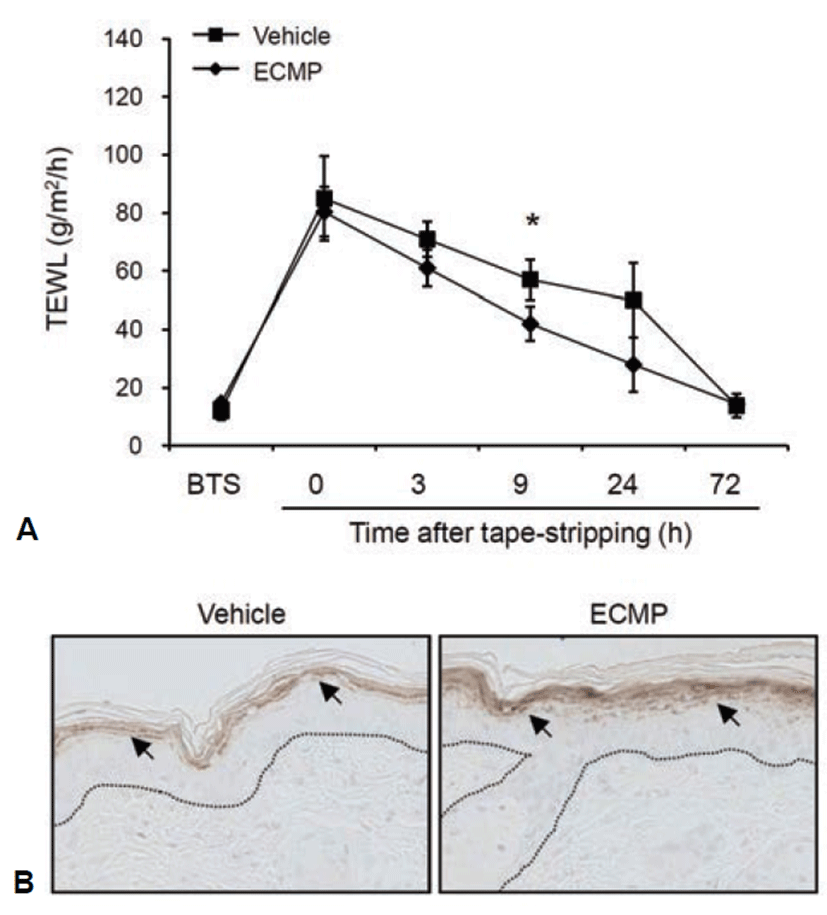
Transepidermal water loss (TEWL) is a well established indicator for the disturbance of skin barrier function. To investigate the effect of ECMP on skin barrier function, we disturbed mouse skin barrier by tape-stripping. We then topically applied ECMP and checked the TEWL. As compared with vehicle-treated group, ECMP led to fast recovery of barrier function (Fig. 4A). To further verify the effect of ECMP, we checked the expression of filaggrin by immunohistochemistry. ECMP treatment led to significant increase of filaggrin in granular layer of mouse skin (Fig. 4B).
Discussion
The primary role of skin is the protection of organism from environmental insults. The physical protective barrier provided by keratinocytes is essential for the maintenance of life. Such an important function is established by the keratinocyte differentiation program, in which many of genes are coordinately expressed in a spatiotemporal manner. The progenitor cells in the basal layer of epidermis move upwardly and differentiation process begins in the suprabasal layers. The keratinocytes are fully differentiated at the uppermost part of epidermis and then provide cornified cells on the surface. Especially, cornified cell envelope, a specialized insoluble structure formed beneath the plasma membrane in fully differentiated keratinocytes, is the most important structure providing barrier functionality [1]. The cornified cell envelope is built by the cross-linking of various proteins including filaggrin, involucrin, loricrin and keratins, which are expressed in a spatiotemporally-regulated manner during keratinocyte differentiation [15]. The defect in keratinocyte differentiation is related with various diseases, such as psoriasis and atopic dermatitis [16, 17]. In this regard, the approach that enhances keratinocyte differentiation thereby normalizing the skin texture is one attractable method for curing the differentiation-related skin diseases. We evaluated the effect of ECMP, which was prepared using traditionally-used medicinal plants, on keratinocyte differentiation. It was demonstrated in the present study that ECMP has a potential for enhancing keratinocyte differentiation and enhancing recovery of disturbed skin barrier.
The mechanism underlying ECMP-induced keratinocyte differentiation is not known. However, we can speculate the possible action mechanism by ECMP, based on the effects of its ingredients. For example, Houttuynia extract modulates G0/G1 arrest in human lung cancer A549 cells [18]. As mentioned before that kerationocyte differentiation incurs cell cycle arrest simultaneously, it is suggestive that Heartleaf Houttuynia in ECMP may affect negatively the cell cycle of keratinocytes, thereby inducing differentiation process. In other example, Glycyrrhiza extract induces expression of cyclin-dependent kinase (CDK) inhibitor p21 in MCF-7 cells [19]. Since involvement of p21 in keratinocyte differentiation has been demonstrated repeatedly [20, 21], it is speculated that Glycyrrhiza uralensis Fischer in ECMP may affect the expression of p21 in keratinocytes, which in turn induces cell cycle arrest and onset of differentiation. The precise action mechanism of ECMP on keratinocyte differentiation, however, should be investigated further.
In summary, ECMP has a potential for enhancing keratinocyte differentiation, suggesting that ECMP may be applicable for keratinocyte differentiation-related skin diseases as a co-modality together with first-line treatments.







