Introduction
patent ductus arteriosus (PDA) is the most common congenital cardiac deformity that is rectifiable, and it is identified by the presence of abnormal shunting vessels between the aorta and the pulmonary artery. It occurs with high prevalence in young female dogs of small breed [5, 9]. Surgical occlusion via thoracotomy is traditional method of treating PDA. However, due to the invasive nature of surgical occlusion, minimally invasive transcatheterial occlusion is becoming more popular [10]. There are three methods of trans-catheterization; occlusion can be achieved by coil embolization or by implantation of an Amplatz® Vascular plug or an Amplatz® Canine Duct Occluder (ACDO). Among them, transarterial coil embolization is the most common method of PDA occlusion, and traditionally, catheterization is performed via the femoral artery in small dogs with PDA. The use of the other two devices for PDA occlusion has been reported in the literature, and especially the ACDO has been developed to address the anatomical differences between canines and humans [1]. This case report presents the successful transarterial occlusion of PDA in a dog using an ACDO of a size that was smaller than the recommended size, which was chosen to accommodate small-sized dogs.
Case report
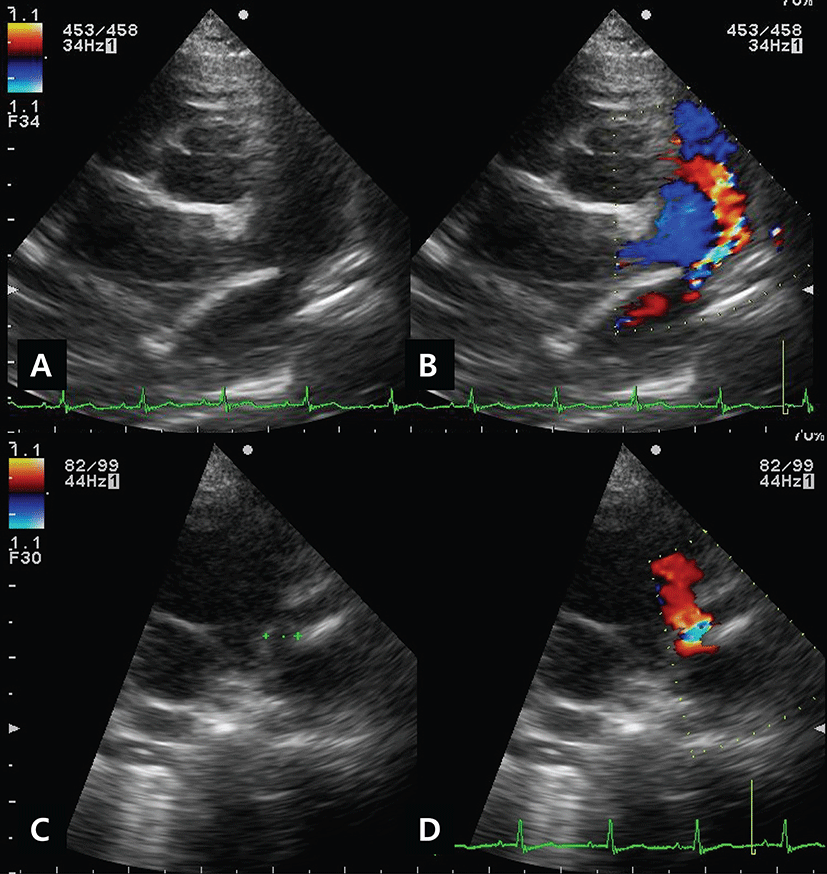
A two-year-old-2.53kg-intact female Pomeranian dog was referred due to a continuous heart murmur and precordial thrill. A 5-days history of dyspnea, cough and exercise-intolerance were also noted by the referring veterinarian. A grade V/VI machinery murmur was auscultated at the left base of the heart. Electrocardiogram revealed tall R waves and widening of the QRS amplitude. Bulging of the main pulmonary artery, pulmonary vein and descending aorta was present on thoracic radiographs. Echocardiography revealed left ventricular and atrial dilatation, and the fractional shortening was decreased (fractional shortening=25.9%, ejection=52.5%). The LA/AO ratio was 1.7 and the mitral regurgitation flow was 4.7 m/s. A structure suspected to be a duct was found (Figs. 1A and 1C), and turbulent flow at the MPA was demonstrated on color-doppler examination (Figs. 1B and 1D). Based on the result of the investigations, the dog was diagnosed with a PDA. For stabilization before the procedure, pimobendan (0.3 mg/kg, twice daily p.o.; Vetmedin®, Boehringer Ingelheim, Meda Manufacturing GmbH; Germany), furosemide (2 mg/kg, twice daily p.o.; Lasix; Handok, Seoul, Korea), theophylline (7.5 mg/kg twice daily p.o.; Theolan-B; Kunwha Pharm, Seoul, Korea) and an angiotensin converting enzyme inhibitor, ramipril (0.125 mg/kg once daily p.o.; Vasotop®P; Intervet International B.V., EU), were administered for one-week before the procedure.
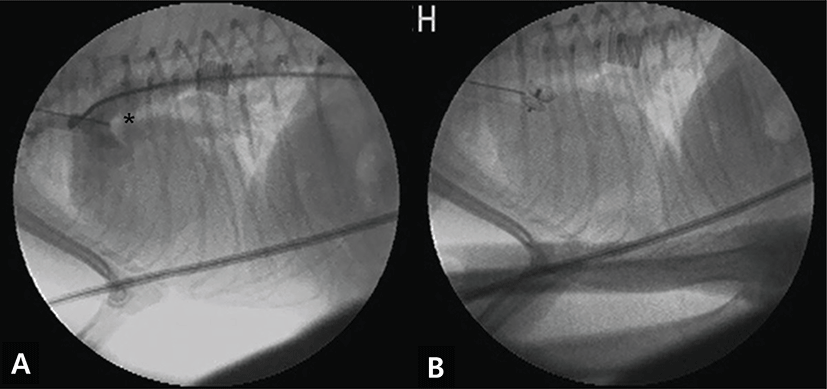
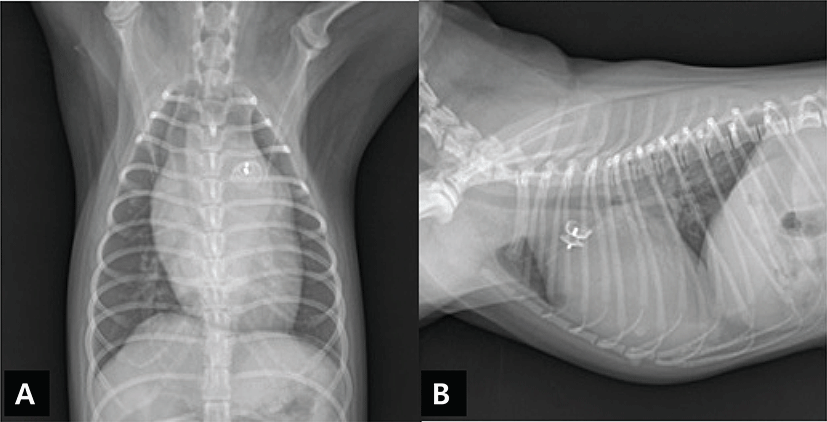
The dog was premedicated with butorphanol (0.4 mg/kg, i.m.; Butophan; Myung Moon Pharm, Seoul, Korea) and acepromazine (0.025 mg/kg, i.m.; Sedaject; Samumedian Co., Ltd, Seoul, Korea). Anesthesia was induced with propofol (6 mg/kg, i.v.; Provive™ 1%; Claris Lifesciences Limited, India) and maintained with 2% isoflorane (Terrell™; Pramal Critical Care Inc., USA) in oxygen. Electrocardiography, body temperature and non-invasive blood pressure recording were used to monitor the anesthesia. With the patient in the right lateral recumbent position, access to the femoral artery was obtained by the surgical isolation method. A 4-Fr vascular introducer (Check-Flo Performer® Introducer sets, Cook, Bloomington, IN, USA) was inserted, followed by 4-Fr angiographic catheterization (Boston Scientific Corporation, MA, USA) into the descending aorta, with fluoroscopic guidance. An angiogram was taken after the injection of non-ionic contrast medium (Omnipaque; GE Healthcare, Milwaukee, WI, USA, 1~1.2 mL/kg), and a Type IIb PDA was seen between the descending aorta and the pulmonary artery, with distal narrowing in the ductal diameter [4]. The minimum ductal diameter was 4 mm (Fig. 2A), and an interventional treatment using a 5 mm transarterial Amplatz Canine Duct Occluder (Amplatz® Canine Duct Occluder; Infiniti Medical, LCC, USA) was selected. For the occlusion, the angiocatheter was replaced by the delivery catheter (Flexor® Check-Flo Performer® Introducer, Cook, Bloomington, IN, USA), which was placed in the descending aorta and then introduced into the ductus. The ACDO was released at least 5 minutes after the proper position was attained (Fig. 2B). After deployment of ACDO, the cardiac murmur disappeared. Postoperative analgesia was administered by an injection of butorphanol (0.1 mg/kg, s.c., Butophan; Myung Moon Pharm, Seoul, Korea) and cefazolin (30 mg/kg, i.v., Korea Chorus Pharm, Seoul, Korea). Thoracic radiography and an electrocardiogram were performed to evaluate the position of the ACDO (Figs. 3A and 3B). The dog was discharged the following day without any complications and pimobendan (0.3 mg/kg, twice daily p.o.; Vetmedin®, Boehringer Ingelheim, Meda Manufacturing GmbH; Germany), furosemide (2 mg/kg, twice daily p.o.; Lasix; Handok, Seoul, Korea), theophylline (7.5 mg/kg twice daily p.o.; Theolan-B; Kunwha Pharm, Seoul, Korea) and ramipril (0.125 mg/kg once daily p.o.; Vasotop®P; Intervet international B.V., EU) were administered for two more weeks before re-examination.
The patient was referred back to the local hospital because of the geographical distance involved in attending this hospital, and to date, there has been no problem for at least 10 months.
Discussion
PDA is the most common congenital cardiac defect that can cause volume overload of the left heart, pulmonary overcirculation and left-congestive heart failure. If uncorrected, mortality due to the PDA within a 1-year period approaches 60% [11]. In human medicine, PDA transcatheterial occlusion is a popular treatment for this condition because of its high suitability for patients of varying ages and body sizes [6, 8]. In veterinary medicine, as previously documented [5, 9, 13], the traditional treatment for PDA has been surgical occlusion. However, various complications such as hemorrhage, recanalization and incomplete ligation can occur. Amongst these, hemorrhage which occurs more commonly with surgical ligation than with transarterial catheterial occlusion, is the most frequently encountered complication and it can be fatal [1]. Over the past decade, transarterial occlusion of PDA has become more common [3, 7, 10]. The benefits of ACDO compared to coil-embolization or vascular-plug occlusion include the ease of deployment, a highly complete occlusion rate, the ability to reposition of the device prior to release, the short duration of the procedure and the rapid postoperative recovery associated with this device [1]. ACDO can be used for large PDA occlusions, and requires a relatively specific delivery system [1, 3, 7, 10, 13]. Small dogs that weigh less than 2.5 kg present a problem for transarterial closure of PDA, because of the limitations of the femoral arterial approach [2].
In this case, an ACDO was selected in place of coil embolization or occlusion with a vascular plug. Owing to the size of the dog’s femoral artery, a 5 mm ACDO was selected. In principle, based on the measured diameter of the PDA, a 6 mm or 8 mm ACDO would have been required (the recommended size of the ACDO: 150~200% of the MDD) [1]. However, in this dog, the size of the duct was too large for occlusion with coil embolization, and as the owner had neglected giving surgical treatment to the dog [2], we conducted the ACDO procedure even though the size of the duct and the ACDO were not a perfect fit. The device was found to be in a stable position on postoperative radiography. No complications have yet been reported.
In conclusion, this case demonstrates that the use of an ACDO that is even smaller (125% of MDD) than the recommended size (150~200% of MDD) can be a safe and valuable treatment option in the transcatheterial occlusion of PDA in dogs.







