Introduction
A tongue mainly consists of lingual muscles with epithelia, lamina propria and complex connective tissues; collagen and elastic fibers. The lingual epithelium has two embryonic origins: the base region is endodermal and the remainder is ectodermal. The lingual simple epithelium of early embryonic life gradually becomes stratified squamous epithelia which are characterized by the presence of larger polypeptides, mainly cytokeratin (CK), but the profile varies with the differentiation program [1, 2].
CK, a hallmark of epithelial differentiation, is the intermediate filament proteins characteristic of epithelial cells. They comprise a gene family of more than 20 different polypeptides in human tissues [2] and different sets of CK molecules are expressed in various epithelial tissues and those at various stages of differentiation [1, 3]. The expression of various CK polypeptides in epithelial cells depends on an organ, the type of epithelium, and the differentiation status of an individual epithelial cell [4, 5]. In human diagnostic histopathology, CK expression can be regarded as an established marker of neoplastic changes and non-neoplastic epithelial lesions [6, 7, 8]. CK expression has also frequently been used in rat models as a relevant marker of epithelial differentiation changes during development [9].
Many studies have been conducted to show the distribution of CKs in adult mice and human [10, 11] and the changes in CK expression pattern in developing mice and human tongues have been described [2, 12, 13].
However, to our knowledge, the changes in CK profiles in the developing goat tongue have not yet been published. We have identified these changes and correlated the results with morphological maturation of lingual epithelium during the development of goat tongues.
Materials and Methods
The tongues of three fetuses were removed on 60, 90, 120-day-old fetuses and neonates originated from 2 to 4 years old Korean native goats (body weight 23 to 33 kg) by caesarean section performed under general anesthesia using xylazine hydrochloride (Bayer Korea Ltd., i.v., 10 mg/kg) and the tongues were examined for morphogenesis during development. All animal experiments were performed according to a protocol set out in the guidelines of the Animal Experiments Ethics Committee at Gyeongsang National University (Approval No. GNU-LA-10).
The tongues were post-fixed for 48 hr and embedded in paraffin wax. The tissue samples were serially sectioned at a thickness of 5~7 μm on a rotary microtome for immunohistochemical analysis of CK distribution patterns. Serial sectioned tissues were mounted on charged slides, and then pre-treated with microwave (800 Watt) in citric acid buffer (pH 6.0) for 20 min. The sections were incubated in 0.3% Triton X-100 (Sigma-Aldrich, MO, USA) and 0.1 M phosphate buffered saline (PBS, pH 7.4) overnight at 4°C and in a blocking solution that contains 10% normal goats serum to reduce non-specific reactions overnight at 4°C. After washing with PBS three times, the sections were exposed to rabbit polyclonal CK (1:50; Invitrogen; CA, USA) antibody in PBS (pH 7.2) containing 0.5% bovine serum albumin for 2 hr at room temperature. The slides were washed again with PBS and incubated using appropriate anti-rabbit biotinylated secondary antibodies for 1 hr at room temperature. The reactions were visualized with avidin-biotin-peroxidase complex kit (ABC, Vector Co) for 2 hr followed by incubation with 3-amino-9-ethylcarbazole (ACE) as a substrate for 10 min. Reacted sections were briefly counter-stained with Meyer’s hematoxylin and then immunoreactive cells were observed under light microscope. Substituting blocking solution for primary antiserum routinely was performed for controls.
Results
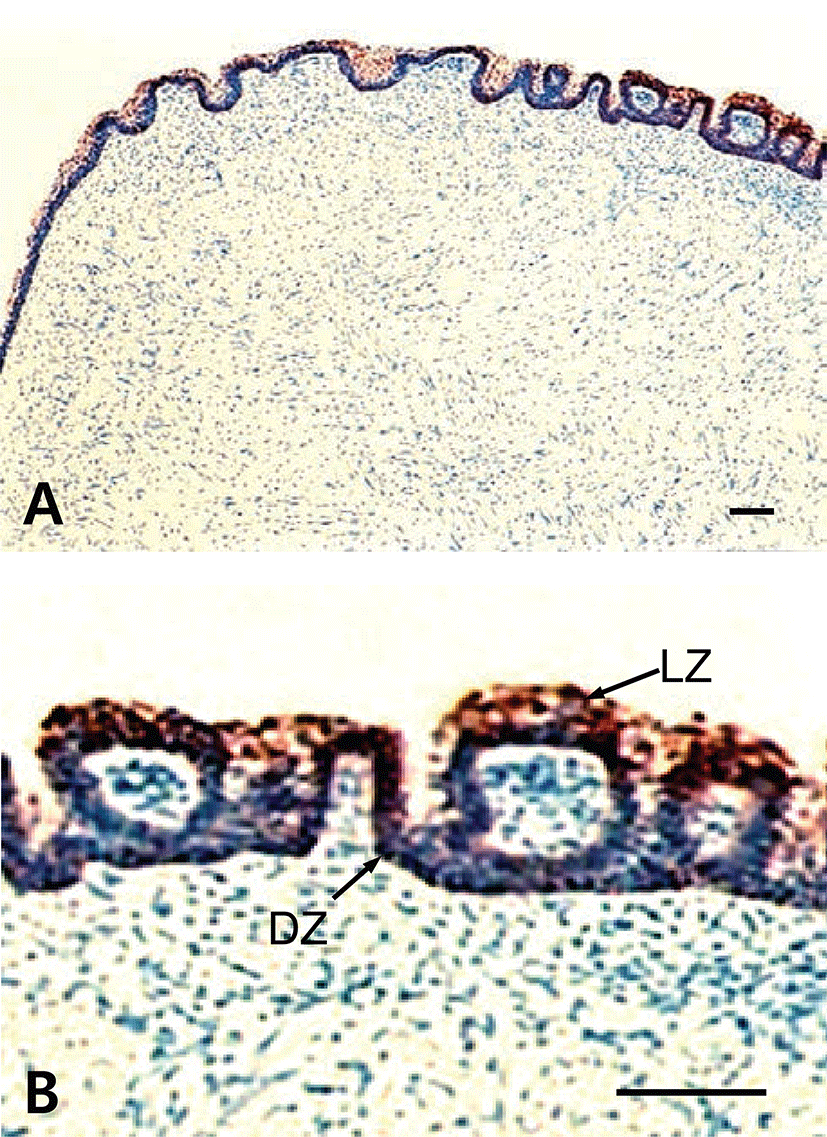
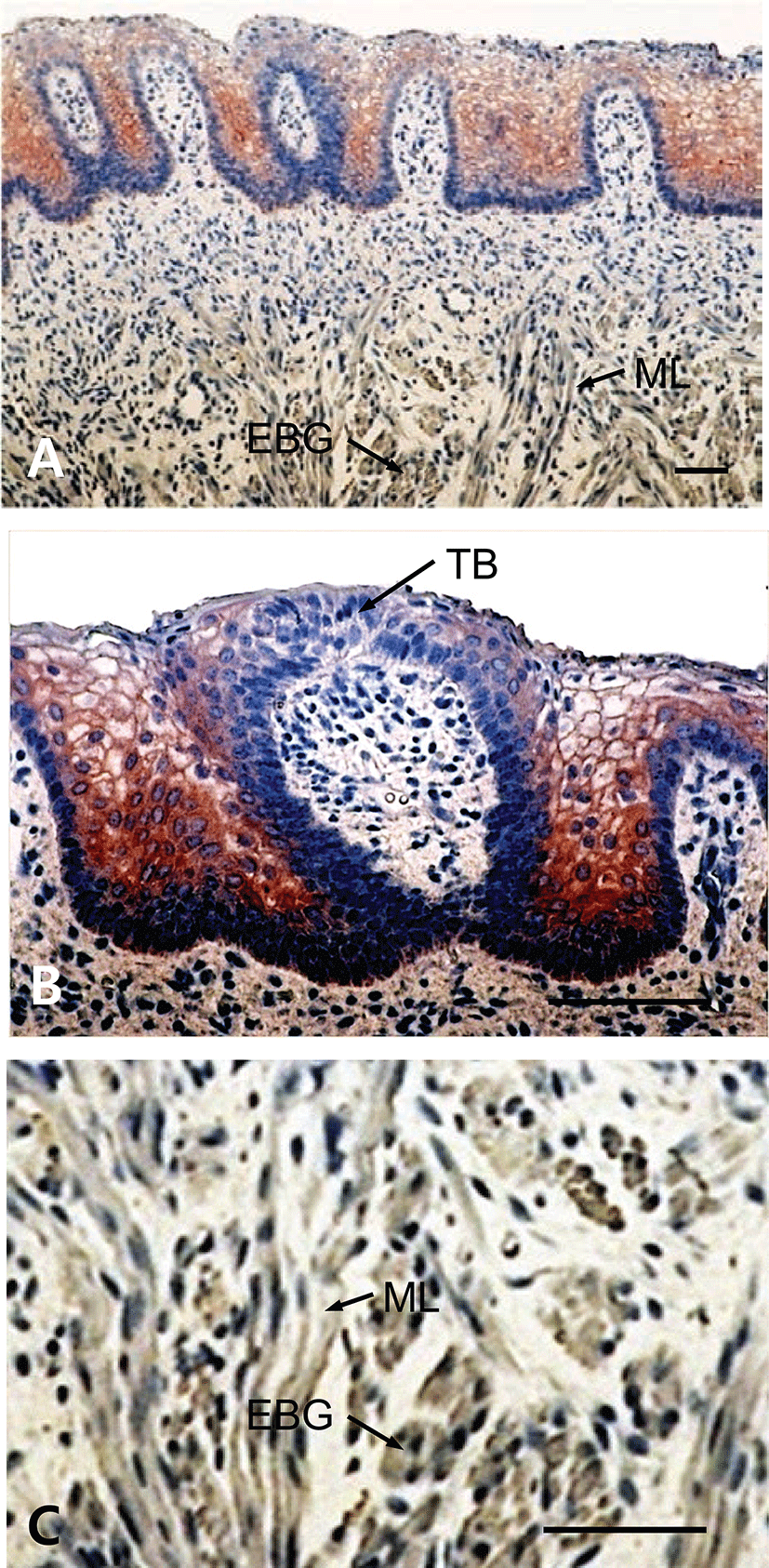
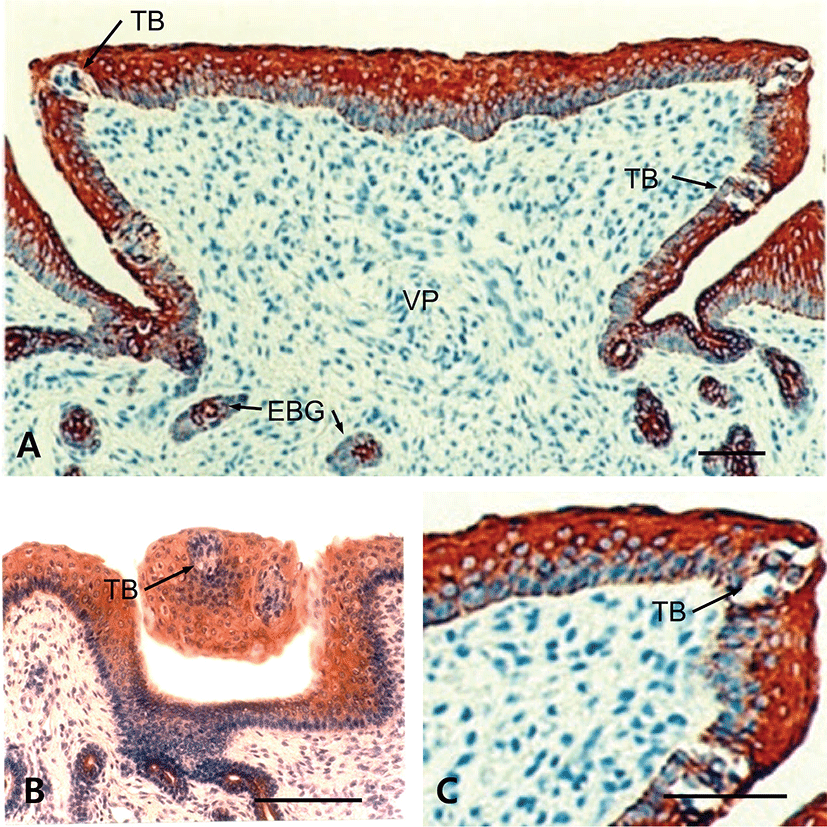
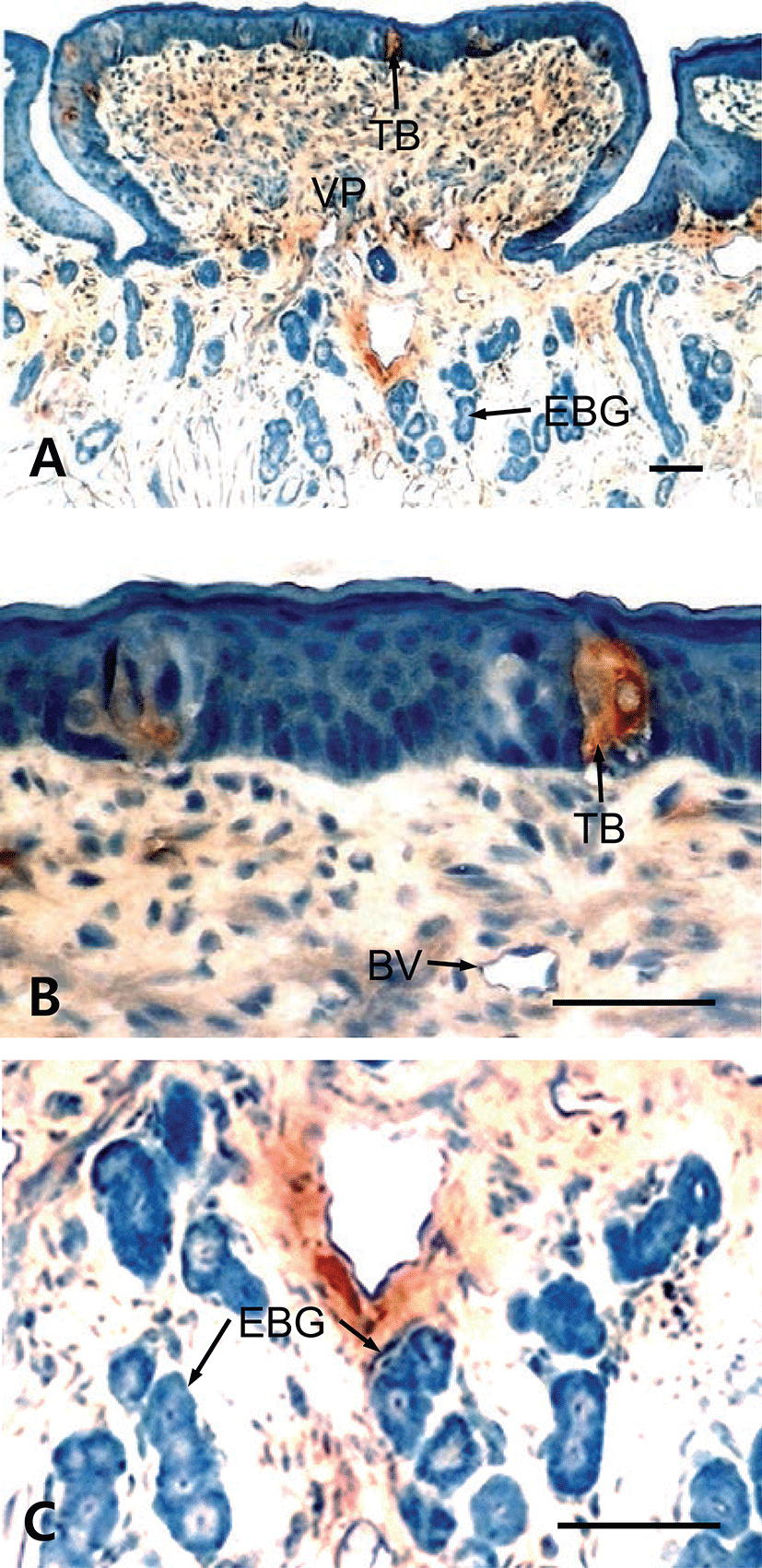
The results showed that tongues of the fetuses on days 60, 90, 120 and neonate were examined for the immunohistochemistry for CK protein during prenatal development. In 60-day-old fetuses, the apical part of light zone of dorsal lingual epithelia was weakly positive to CK-immunoreactivity (Fig. 1). The light zone of an intermediate part in dorsal lingual epithelia consisted of 3 to 4 layered stratified cells (Fig. 1B). In 90-day-old fetuses, the light zones of deep areas in dorsal lingual epithelia were strongly positive for CK and superficial areas were moderately positive, whereas von Ebner’s glands were showed negative for CK (Fig. 2C). The epithelium of lingual papillae consisted of 4 to 8 layered stratified cells (Fig. 2B). In 120-day-old fetuses, the light zones of lingual epithelia in the vallate papilla were strongly positive for CK, whereas ducts of von Ebner’s glands were moderately positive (Fig. 3A). The taste buds in the vallate papilla were weakly positive for CK (Figs. 3B and 3C). In neonates, taste buds in the vallate papillae were moderately positive for CK (Figs. 4A and 4B), whereas non-taste epithelial cells and von Ebner’s glands in the tongues were negative for CK expression (Figs. 4B and 4C).
Discussion
There are many reports of three-dimensional structures of lingual surface of tongues in mammals based on scanning electron microscopy (SEM). Especially, a number of light microscopic and SEM studies have been conducted with developing goat tongues [14-16]. However, there has been no study on immunohistochemistry for CK in the lingual epithelium of tongues in developing Korean native goats.
The tongue is covered by a complex mucosa exhibiting diverse characteristics. To clarify intermediate filament patterns of tongue mucosa and lingual gland in goat from 60-day-old fetuses to newborn during development, the expression patterns of CK were investigated immunohistochemically, using antibody for CK protein in this study.
Cytoskeleton is divided into microfilament, intermediate filament and microtubule. Intermediate filament has tetramers helix structure composed of extended filamentous monomers, which is 8 to 10 nm in diameter. The intermediate filament is able to subdivide into 5 kinds, and these intermediate filaments have been used in determining cell morphogenesis origin by CK. Meanwhile, studies on cytoskeleton have been allowed to reveal the origin of several tumor cells, which is in turn useful in the early diagnosis of cancer [17, 18]. CK have been used as an important marker of the development, degeneration, regeneration, and cell turnover. Moreover, it is very useful in observing the development and differentiation of lingual epithelial cells of rat [19, 20, 21], mouse [12], and human [2, 13]. Takeda et al [12] reported that Cytokeratin-Immunoreactivity (CK-IR) appeared on the epithelia of vallate papillae of 17 to 18-day-old mouse fetuses and immature basal cells in the taste buds of newborns. In this study, CK-IR was weakly to moderately positive in the light zone of the lingual epithelium in 60- to 90-day-old fetuses, respectively.
In the developing human tongue, CK-IR appeared in taste buds and von Ebner’s glands, but the appearance of CK-IR in human lingual epithelia gradually decreased according to getting age. In addition, CK-IR appeared on epithelial cells, base cells, and perigemmal cells at the early stage of development. On the other hand, it only appeared in taste bud by getting age [13]. We demonstrated that the taste buds of vallate papillae in neonates revealed strongly positive for CK, whereas von Ebner’s glands were negative in neonates, suggesting that CK can be a useful marker to find the appearance of taste buds in developing tongue.
Bradley and Stern [22] reported that the lingual epithelia of 6 to 7-week-old human fetuses consisted of superficial and deep layer and the epithelia of it were composed of 2 to 3 cell layers. The lingual epithelia of 20-week-old human fetuses formed stratified squamous epithelia consisted of 10 to 15 layers. In this study, the tongue of 60-day-old fetuses was already composed of epithelia which were covered with stratified squamous epithelia. Especially, the light zones of epithelia were positive for CK. We previously demonstrated that rudiments of taste buds appeared on fetal goat tongues as early as 60 days of gestation and taste buds may have acquired adult morphological characteristics by newborns [14]. Therefore, different patterns of CK expression during development in goat tongues may take place concurrently with structural development.







