Introduction
Ischemic stroke is a leading cause of neurologic disability and human mortality worldwide. Transient or permanent occlusion of affected cerebral arteries can lead to a substantial reduction of blood flow in the territory of the affected arteries and cause cerebral ischemic stroke [1]. In addition, the subsequent proinflammatory cascade can lead to permanent neurologic deficit or severe disability. Although there are various animal models of ischemic stroke, most of them are small animal models (e.g., mice, rats, and gerbils). Compared with those animals, larger animals, including dogs, have brain and vasculature anatomy more similar to those of humans (e.g., gyrencephalic brains and substantial white matter among other similarities). Accordingly, larger animals may be more appropriate as ischemic stroke models [1, 2].
Magnetic resonance imaging (MRI) is considered an essential diagnostic device for evaluation of patients with symptoms suggestive of ischemic stroke. Especially in cases of acute stroke, MRI is not only superior to computed tomography for detection of ischemic lesions, but for differentiation of acute one from chronic hemorrhage [3]. Another advantage of MRI is its ability to differentiate stroke from other conditions that present similar focal and sudden-onset neurologic signs [3]. These advantages make MRI an ideal diagnostic tool for ischemic stroke in human medicine [3]. Postmortem examination and histopathologic study should also be performed for definite diagnosis. The purpose of this study was to describe the neurologic signs, MRI features, and histopathologic findings of experimental acute ischemic stroke in dogs.
Materials and Methods
The study population included 3 healthy mongrel dogs (2 males and 1 female, about 3~5 years old, weighing 8.1, 8.5, and 9.5 kg, respectively). All 3 dogs were physically normal, with no history of neurologic disorders. Results of physical and neurologic examinations prior to the surgical procedure were all normal. Complete blood count and serum chemistry profile results were used as screening tests for metabolic diseases; they were found to be normal. The surgical procedure and experimental protocols, including euthanasia, were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University.
Twenty-four hours prior to surgery, 5 mL of venous blood was obtained from the jugular vein and drawn into a plain tube. After 24 hr of coagulation at room temperature, the supernatant was decanted. A micropipette was used to quantify 200 μL of the autologous thrombus. A prepared 30 mL syringe was then filled with a mixture of quantified autologous thrombus and 20 mL of physiologic saline.
All dogs were made to fast for >12 hr before induction of general anesthesia. Atropine was administered as a premedication 10 min before induction, and anesthesia induction was performed using orally intubated propofol. Isofluran at 2~3% of the inspired volume was used to maintain general anesthesia during surgical procedures. The heart rate, body temperature, and blood oxygen saturation level were monitored and maintained within normal ranges during surgery. Ischemic stroke was induced by using the prepared autologous thrombus. The dogs were placed in lateral recumbency on the operation table, and the cervical area of each dog was sterilized by using alcohol. After making a cervical incision, the common carotid artery and internal carotid artery (a branch of common carotid artery that supplies the anterior part of the brain) were exposed. A 200-μL injection of the autologous thrombus was delivered with a 20-gauge venous catheter through the internal carotid artery. After successful delivery of the autologous thrombus, the venous catheter was removed and the cervical incision was sutured.
MRI was performed 1 day before and 1 day after induction of ischemic stroke with a 0.4T magnet MRI system (APERTO 0.4, Hitachi Medical Co., Tokyo, Japan). Under general anesthesia, transverse T1-weighted imaging, T2-weighted imaging, FLAIR imaging, postcontrast T1-weighted imaging, and diffusion-weighted imaging (DWI) were performed (Table 1).
A day after induction of ischemic stroke, MRI results were obtained, and all dogs were euthanized. The brains were carefully removed and dissected into 2 mm coronal slices. The fresh brain slices were immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, MO) in normal saline at 37°C for 30 min, as previously reported [4, 5]. Brain slices were also immersed in 10% paraformaldehyde in phosphate buffer for at least 72 hr for fixation. After immersion fixation, the slices were dehydrated and embedded in paraffin. Transverse sections (5 μm) were cut. At the end of staining with hematoxylin and eosin, light microscopy was used to detect any histopathologic alterations caused by ischemic stroke.
Results
Physiologic parameters before, during, and after surgery were well maintained until euthanasia. To prevent infection and pain after surgery, antibiotics and analgesics were administered until euthanasia. Neurologic signs, including generalized seizures, tetraparesis, and altered mental status, were observed in all 3 dogs after induction of ischemic stroke. These neurologic signs manifested immediately after awakening from anesthesia.
MRI findings before ischemic stroke induction were all normal; however, 1 day after ischemic stroke induction, MRI revealed multifocal lesions in the cerebral cortex and subcortex, with T1 hypointensity, T2 hyperintensity, FLAIR hyperintensity, and DWI hyperintensity in all 3 dogs (Fig. 1). Affected lesions were well differentiated from the adjacent normal brain parenchyma. Mild deviations of the falx cerebri caused by acute cerebral edema formation and mass effects were also identified (Fig. 1).
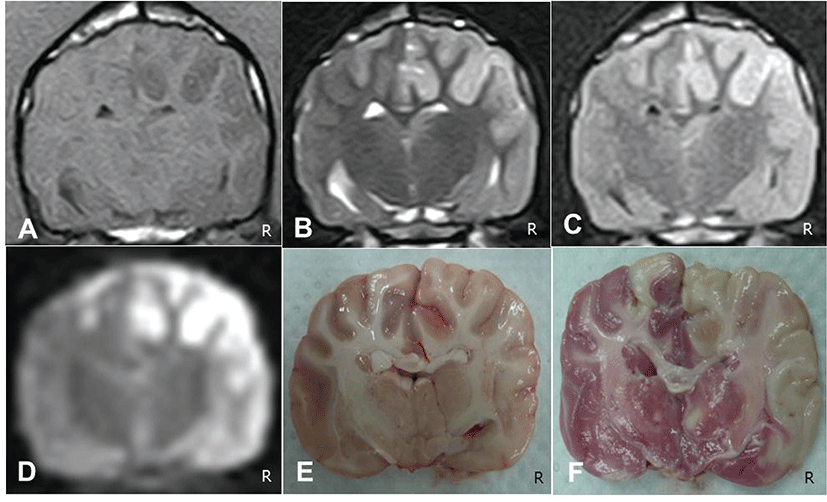
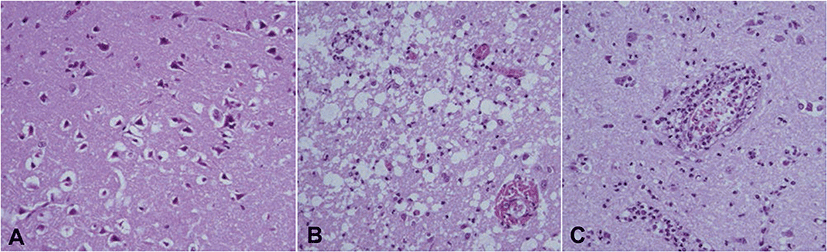
No remarkable changes were identified in gross postmortem examination of the 3 dogs; however, the coronal brain slices showed edematous lesions, which obscured border lines between the gray and white matter (Fig. 1F). In addition, mild deviations of the falx cerebri due to acute cerebral edema formation were identified (Fig. 1F). These affected lesions were not stained with TTC, in contrast to the red-stained normal brain parenchyma (Fig. 1E). Histologic features of the earliest neuronal changes, such as cytoplasmic eosinophilia with pyknotic nuclei, were identified. Neuropil spongiosis and perivascular cuffing were also prominently observed at the infarcted area (Fig. 2).
Discussion
Conventional MRI sequences, including T1- weighted, T2-weighted, and FLAIR images, are only marginally useful for diagnosing ischemia within hours of onset because they are sensitive to vasogenic edema, a process that develops gradually over time. Identification of vasogenic edema with conventional MRI sequences may be difficult within the first 24 hr of stroke [4, 6, 7, 8]. In addition, during the first 24 hr after ischemic stroke, conventional MRI gives false-negative results 20% to 30% of the times [9, 10, 11]. This false-negative probability increases to 30% to 50% during the first 3 to 6 hr [12, 13]. In contrast, DWI sequences can detect ischemic lesions within minutes of onset. Signal changes on DWI sequences reflect the relative restriction of water diffusion due to influx of water from the extracellular to the intracellular space [4, 8]. Therefore, even at very early time points, DWI is highly sensitive and specific for acute ischemic lesion identification. In this study, however, ischemic lesions were also identified easily on conventional MRI sequences 1 day after ischemic stroke induction. Results of conventional MRI taken 1 day after induction of ischemic stroke were almost identical to the results of DWI. Based on these findings, conventional MRI sequences could also be a useful diagnostic tool for diagnosing acute to subacute ischemic stroke. Additionally, specific neurologic signs after ischemic stroke are associated with the location and size of the ischemic lesions [14, 15]. Observed neurologic signs in this study included generalized seizures, tetraparesis, and altered mental status. Of these, generalized seizures are considered one of the most common signs of forebrain disease [14, 16]. Observed lesions in this study were mainly located on the cerebral cortex and adjacent subcortex area of the forebrain. Those lesions were also identified on TTC staining and histopathologic examination. Histopathologic findings in the canine model revealed typical features of acute ischemic stroke such as hypoxic neuronal changes, neuropil spongiosis, and perivascular cuffing. In this study, we used clots derived from unmodified autologous blood to induce ischemic stroke. There were two main forms of clots; one was derived from unmodified blood [17], and one was derived from blood mixed with thrombin [18]. Although both types seemed to provide similar levels of occlusion, thrombin-induced clots appeared more resistant to the effects of tissue plasminogen activator as a thrombolytic therapy [19]. Because thrombolytic therapy within 3 to 6 hr of the onset of ischemic stroke is effective in restoring blood flow and improving stroke prognosis in humans [20], unmodified autologous blood clots are more suitable for studying thrombolytic therapy in acute ischemic stroke [2, 19].
In the present study, we described the induction of ischemic stroke in dogs with autologous thrombus. Characteristic features of ischemic stroke on MRI, histopathological analysis, and TTC staining were also investigated. On MRI, T1 hypointensity, T2 hyperintensity, FLAIR hyperintensity, and DWI hyperintensity lesions were consistent with MRI features of previously reported ischemic strokes in humans. Observed neurologic signs in this study were also consistent with reported effects of ischemic lesions. Based on the results of this study, further studies with highly sophisticated pathophysiologic processes and advanced imaging modalities would be helpful for the diagnosis and treatment of ischemic stroke in dogs.







