Introduction
Feline immunodeficiency virus (FIV) belongs to genus Lentivirus, the Retroviridae family and is an important pathogen of domestic cats [1]. FIV is distributed worldwide with a high incidence in free-roaming cats living in multi-cat households [1, 2]. The major route of transmission is through bite wounds, which inoculate virus-laden saliva into the naive recipient [2, 3]. The virus disrupts immune system primarily by infecting T-lymphocytes, B-lymphocytes, and macrophages [2, 3]. Clinical signs of the infected cats result from progressive disruption of immune function, which increases susceptibility to secondary infections, immune-mediated disease, and neoplasm [2-5]. Diagnosis of FIV infection is confirmed using a PCR assay to demonstrate the proviral DNA and/or an ELISA to detect the associated antibodies [3]. Treatments of the FIV-infected cats consist of antiviral therapy and immune function modulators. Approved medications in veterinary practice for naturally infected cats with FIV include azidothymidine (AZT) [2, 6] and interferon alpha (IFN-α) [7, 8]. Several treatment trials have been attempted in experimentally FIV infected cats; however, few studies have evaluated naturally infected cats [2]. This case report describes the clinical outcomes in a naturally FIV-infected cat treated with a combination of antiretroviral agents-AZT and IFN-α.
Case report
A 7-year-old, spayed female, domestic short hair cat was referred for evaluation of a 2-week history of anorexia, depression, and weight loss. The cat was previously adopted from an animal shelter in Hong Kong and resided in Japan for 2 years primarily in an outdoor environment. Waxing and waning clinical signs of illness were first noted at 3 years of age and recurred 2 years later. On presentation, the cat was cachexic. Physical examination showed pyrexia (39.9°C), mild gingivitis, and pale mucus membranes. A complete blood count (CBC) revealed mild normocytic normochromic non-regenerative anemia (packed cell volume [PCV], 22.14%; reference range, 24.0~45.0%) and severe thrombocytopenia (13 × 103/μL; reference range, 150~500 × 103/μL) (Fig. 1). Serum biochemistry revealed mild hyperproteinemia (total protein 8.7 g/dL; reference range, 6.6~8.4 g/dL) resulting from hyperglobulinemia (albumin/globulin ratio 0.4; reference range, 0.8~1.68). A serum protein electrophoresis displayed elevated alpha-2 fraction within the globulin concentration (2.7 g/dL; reference range, 0.4~0.9 g/dL). Based on the history, clinical signs, and laboratory results, systemic viral infection was strongly suspected. Total RNA was extracted from whole blood sample using viral DNA/RNA extraction kit (Intron), according to the manufacturer’s protocol and was amplified using a Maxime RT-PCR PreMIX (Intron), according to the manufacturer’s protocol with the primers which were designed based on the env gene of FIV ujsing the sequences obtained in GeneBank (NC_001482). The forward primer was 5’ AAA ATG TGG ATG GTG GAA TCA AA 3’ (7321 bp~7343 bp) and the reverse primer was 5’ AGA TGT GCA AGA CCA ATT TCC AG 3’ (7851 bp~7873 bp). The product size of RT-PCR was expected 553 bp.
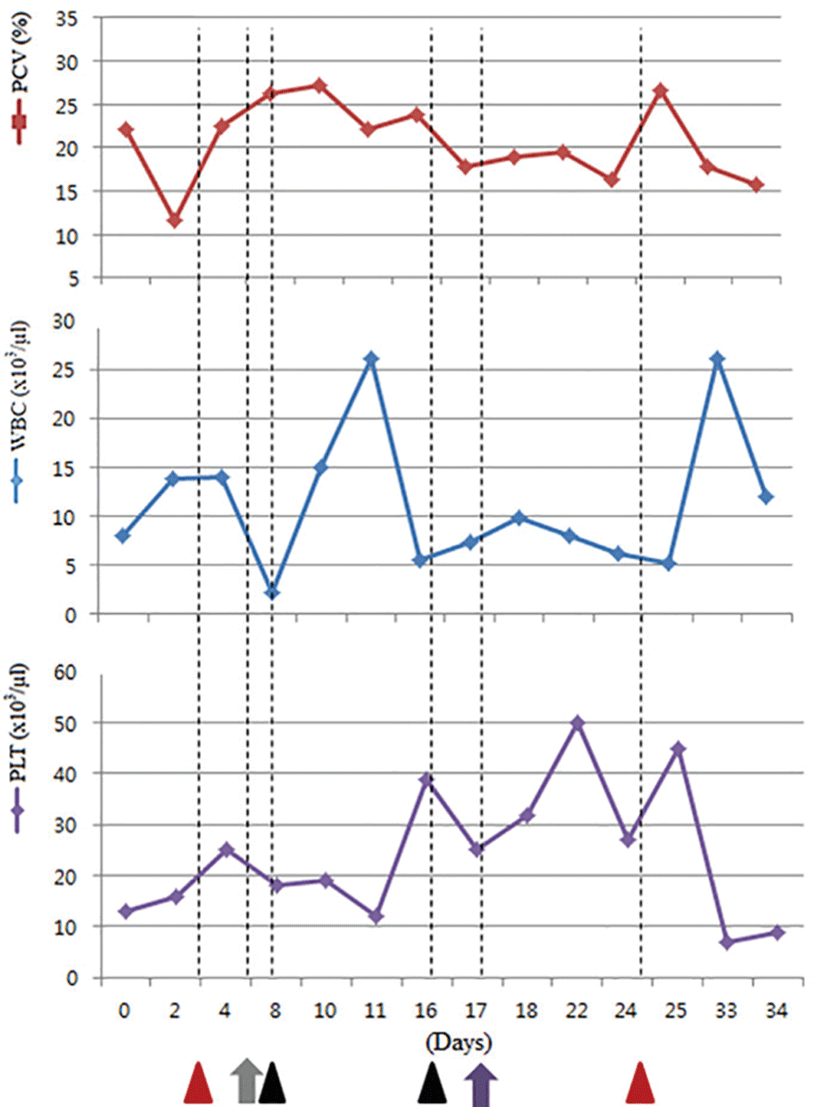
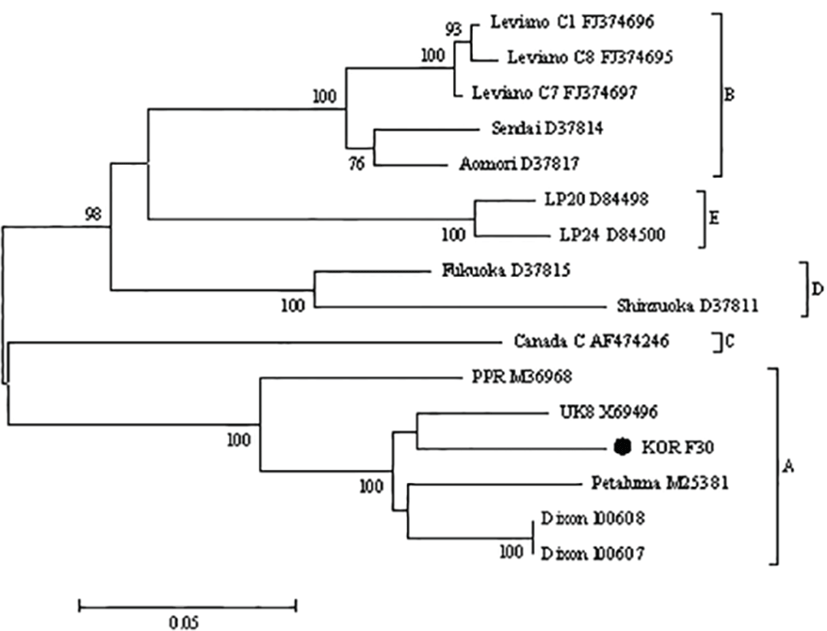
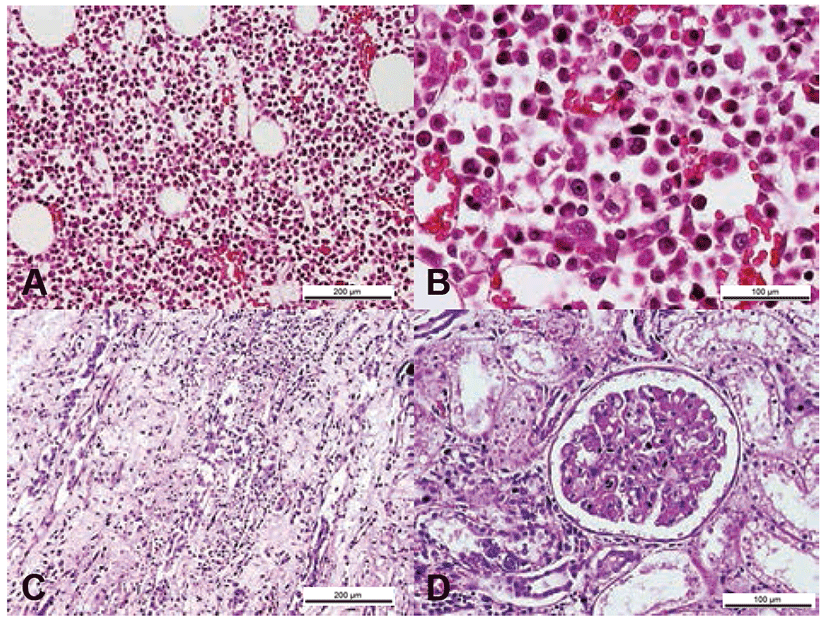
Gene sequencing of the variable gp100 region of env gene identified the virus as FIV subtype A (Fig. 2). As the cat was also seropositive for FIV on ELISA (ANTECH Diagnostics®, MS, USA), it was diagnosed as an FIV infection. Treatment with the antiviral agents AZT (Azidomine; Chung Gei pharm, Korea; 5 mg/kg, PO, BID) and recombinant human IFN-α (Intermax gamma; LG life sciences, Korea; 30 mg/kg, PO, SID) was initiated and continued for 4 weeks. Despite these efforts, the PCV gradually decreased to 16% (Fig. 1). A blood transfusion was performed but provided only a temporary benefit, and the PCV ultimately declined to 12% followed by leukopenia. The cat’s condition progressively declined owing to renal failure diagnosed by blood panel and urinalysis, ultimately leading to its death. Necropsy showed petechial hemorrhages on the oral mucosa and hepatic and bilateral renal necrosis. Histopathology revealed hypercellularity within the bone marrows, which comprised primarily myeloid precursor cells (Fig. 3). In this case, advanced FIV infection caused secondary organ dysfunction and chronic wasting syndrome resulting in death.
Discussion
The cat in this case developed waxing and waning clinical signs of illness after 1 year of outdoor housing in Japan. According to the phylogenetic analyses of gp100 region, the virus in this case was subtype A, which is a common type in Japan [1, 9]. Based on clinical epidemiology, this cat was most likely infected while residing in Japan.
There are four defined clinical stages of FIV infection. The primary phase is characterized by transient fever, neutropenia, lymphadenopathy, conjunctivitis and gingivitis. In most of cases, this stage is transient, subclinical, and unnoticed; the following second phase is a prolonged latent period lasting for years. The third phase is characterized by varying signs of illness including recurrent fever, leukopenia, anemia, lymphadenopathy, and chronic progressive oral infections. Finally, cats in the fourth phase suffer from opportunistic infection, myeloproliferative disease, neoplasm, and neurologic disorders [2]. In the present case, the cat first developed clinical signs of fever and conjunctivitis at 3-year-old, followed by a 4-year latent period. At presentation, the cat showed a non-regenerative anemia, leukopenia, recurrent fever, and stomatitis. Therefore, FIV infection in this cat was classified as stage 3. Statistically, the lifespan of cats with stage 3 FIV infection is between 6 months and several years [2].
However, this cat survived only 2-months after the diagnosis and treatment, despite of the aggressive antiretroviral therapy. Necropsy and histopathology revealed petechial lesions in the right kidney and glomerulonephritis. In FIV-infected cats, renal abnormalities are common and include glomerulosclerosis and severe tubulo-interstitial lesions [3]. In particular, petechial lesions, which are usually accompanied by glomerulonephritis in FIV-infected cats, were also observed in the right kidney of this cat. Histopathologically, the bone marrow primarily comprised myeloid precursor cells, which resulted in hypercellularity. As described previously [3], the majority of symptomatic cats experience hypercellularity caused by hyperplasia of individual cell lines, maturation abnormalities, and areas of necrosis, all findings that were observed in this case.
The common treatments for FIV infection are AZT and recombinant human IFN-α [6, 7]. AZT has a virostatic effect and interrupts the reverse transcription of viral RNA genome into proviral DNA [6]. Recombinant human IFN-α is thought to have two modes of action. Chief among them is the modulation of the innate immune system, which allows better control of environmental pathogens and early regulation of immunopathologic conditions [9]. This activity is likely caused by effective control over the inflammatory cytokines present in diseased organs. The presented cat received both AZT and recombinant human IFN-α for two weeks; however, there was no improvement in clinical conditions including anorexia, anemia, leukopenia, and thrombocytopenia. The cat also received a blood transfusion and granulocyte-colony stimulating factor (G-CSF) as supportive therapies, but still did not show any clinical improvement. The quantity of infected virus can affect therapeutic efficacy [6]; the quantity of viral particles and replication in this cat may have exceeded the therapeutic threshold for AZT. In addition, AZT-resistant variants may have been present. The poor therapeutic response was observed late in the course of disease; therefore, it is difficult to determine whether a resistant variant was a factor in this cat. Lastly, although IFN-α is effective in reducing antigenemia in cats, continuing therapeutic benefits may be limited if neutralizing antibody to IFN-α develops [8]. Significant IFN-α antibody titers occurred between 3 and 7 weeks post-administration of recombinant IFN-α [8]. In the present case, IFN-α was administered for 4 weeks, raising the possibility of neutralizing antibody development during the period.
To the best of our knowledge, this report is the first to describe the clinical intervention of naturally infected FIV in cats using AZT and IFN-α and molecular subtype of FIV in Korea. Also, this report suggests the importance of treatment initiation of these treatments in an early phase of FIV that could be effective for the virus.







