Introduction
Chronic kidney disease (CKD) is defined as a clinical entity associated with primary renal dysfunction that has persisted for months to years [1]. CKD is newer term to replace the term chronic renal failure that the disease has a spectrum of severity from asymptomatic kidney to end stage uremia [1].
According to the report described previously [2], the prevalence of CKD is proportionate to the age. The 15% dogs which is older than 10 years have being affected CKD. It is characterized by the development of variably progressive irreversible intrarenal lesions, resulting in impairment of renal excretory, biosynthetic and regulatory function [4, 6]. Although loss of nephrons and secondary progressive impairment of renal function are irreversible in dogs with CKD, appropriate treatment can improve rate of progression, clinical signs and quality of life [5, 6].
Based on the results described earlier, probiotic bacteria are commonly defined as live microorganisms, which, when administered in adequate amounts, confer a health benefit to the host [8, 9]. It contains multiple strains of beneficial bacteria for balancing the digestive tract (Bifidobacterium bifidum, Lactobacillus fermentum, L. acidophilus, L. casei, Enterococcus faecium, L. plantrum, Pediococcus acidilacticii). Uremic toxins diffuse passively from the blood into the lumen of the gastrointestinal tract. It is believed that urease-producing probiotic species hydrolyze urea and maintain a concentration gradient that favors diffusion of urea from the blood to the gastrointestinal tract lumen [9]. Therefore oral probiotic dietary supplement instilled into the gastrointestinal tract could help to convert accumulated nitrogenous wastes into nontoxic compounds [8, 9, 10].
The aim of therapies for CKD is to alleviate clinical signs associated with uremia, minimizing imbalances of electrolyte, vitamin and mineral, support nutrition and prolongation of life span. The therapeutic approach consisted of several evaluations of patient’s historical, physical and diagnostic findings.
Case report
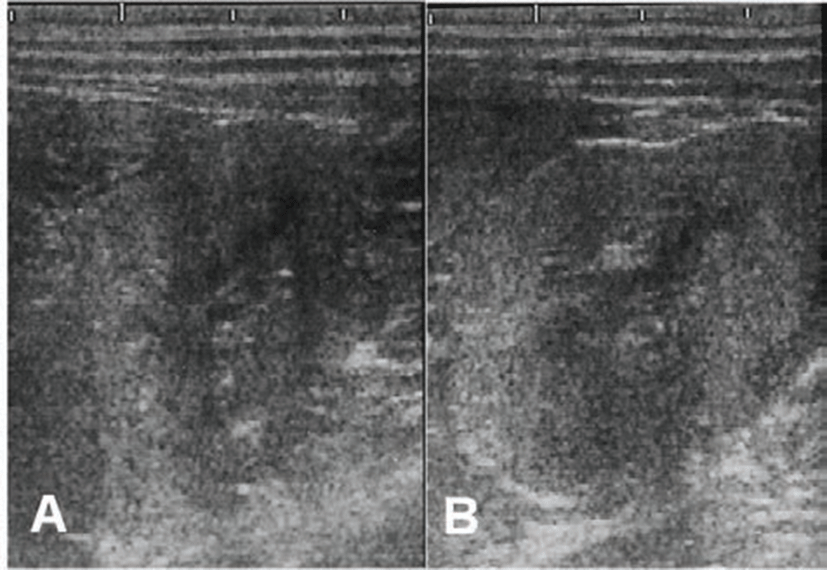
A 7-year-old spayed female English Cocker Spaniel dog was presented with PU, PD and intermittent vomiting. Physical examination revealed pale, tacky mucous membranes and severe emaciation. The body condition score (BCS) of the dog was 2/9 at initial presentation. The dog’s systolic blood pressure was 137 mmHg. Results of the complete blood count revealed normocytic normochromic non-regenerative anemia (Packed cell volume; 27%, reference; 37~55%). Serum biochemical profiles revealed moderate azotemia (Blood Urea Nitrogen; 153 mg/dL, reference; 8~26 mg/dL, creatinine; 4.3 mg/dL, reference; 0.5~1.3 mg/dL) and hyperphosphatemia (phosphorus; 9.5 mg/dL, reference; 3~6.2 mg/dL). Urinalysis showed isosthenuria (urine specific gravity; 1.010). Abdominal ultrasonography revealed bilaterally small lumpy-bumpy kidneys with hyperechoic parenchyma and loss of renal corticomedullary junction (Fig. 1). Based on the clinical signs, physical examinations and laboratory findings, the diagnosis of the dog was CKD. According to the staging system of International Renal Interesting Society (IRIS), This case was made IRIS stage III CKD. Metabolic and inflammatory disease like secondary pancreatitis and urinary tract infection was ruled out by white blood cell (WBC) count, serum lipase level, urine cytology and ultrasonographic examination on abdomen.
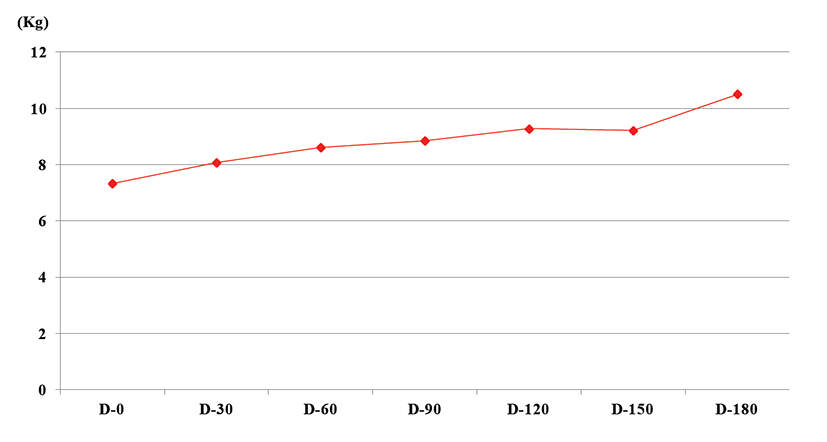
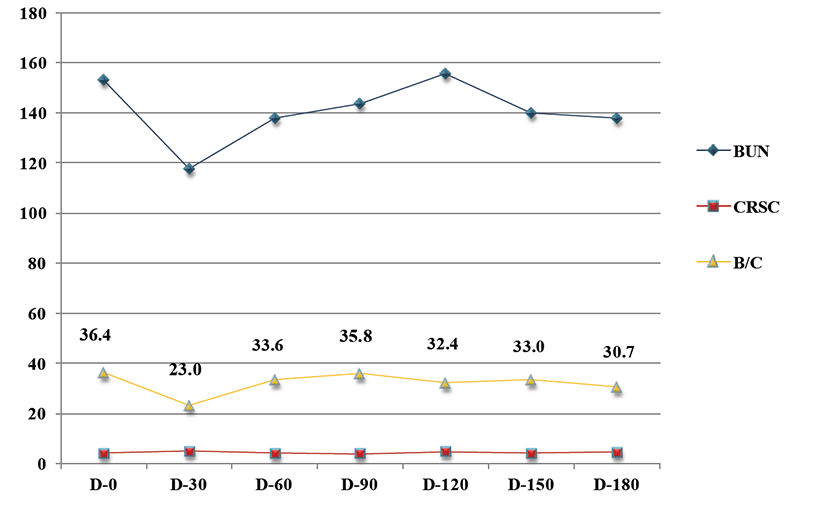
After definite diagnosis was made, the dog was hospitalized and received supportive care with fluid therapy, phosphate binding agent (sevelamer hydrochloride, Renagel® tablets, Genzyme corporation, 400 mg/dog with meal, sid), and histamine H2-receptor antagonist (ranitidine, Nelson, 2 mg/kg, tid) for 3 days. Darbepoetin Alfa (NESP, Kyowa hakko kirin corp. 0.45 μg/kg, sid) was administered to control renal secondary anemia and low protein and phosphate diet (K/D™, Hill’s prescription diet) was fed. Also, dietary probiotics (Bene-Bac Pet power®, Nestle Purina, 2 level teaspoons/dog, sid) was supplemented. Three days later, the dog received treatment as an outpatient. During 6-month treatment period, the patient’s weight has gradually increased (Fig. 2). The BCS was improved 2/9 to 5/9. But PU/PD was not improved. Normal values of acid base balance, electrolyte and blood pressure were identified on initial examination and maintained for 6 months. Because of serum blood urea nitrogen (BUN) and creatinine level were decreased after 30 days later, probiotic supplement medication was discontinued. On 90 days, clinical sign was not deteoriated. However BUN and BUN/creatinine level were elevated, oral probiotic supplement medication resumed without fluid therapy. 2month later, BUN and BUN/creatinine level improved (Fig. 3). The correlation of BUN and probiotic supplement was supposed to positive effect.
Discussion
In general, CKD in dogs is an refractory and irreversible disease with high morbidity and mortality. After diagnosis of CKD has been made, basically, it is important for owners to know that CKD has a progressive and irreversible nature in pathophysiology. Naturally occurring CKD in the dog is a chronic wasting disease, resulting in uremia and death [7]. The concerns of owners with CKD dogs and cats are estimation of life span and therapeutic measures that might increase life span while ensuring comfort and quality of life for their pet [3]. According to the previous reports, despite its irreversible nature, proper treatment of CKD can alleviate clinical signs and slow progression [3, 4, 5]. Thus, conservative medical management of CKD consists of supportive and symptomatic therapies designed to correct abnormalities in fluid, electrolyte, acid-base, endocrine and nutritional balance. In general, therapy in CKD aims to minimize the clinical and pathophysiological consequences of reduced kidney function [5].
An individualized therapeutic approach has to be designed and applied to each dog. General goal for management of CKD is retarding the progression of CKD, through reducing uremic signs due to azotemia, providing optimal nutrition with Vitamin D replacement [7].
Recently many researchers believed that the dietary modification such as probiotic supplementation in CKD dog increases survival time with reduced uremic crisis. Thus most veterinary renal diets are generally restricted in protein, phosphorus, calcium and sodium while supplemented with carbohydrates, sources of alkali (potassium citrate) and poly unsaturated fatty acid in a favorable ratio of omega-6: omega-3 fatty acid [3].
Anemia with non-regeneration and hypoproliferation is characteristic of dogs with moderate to advanced CKD and proportional to the severity of the kidney disease [1]. It may be due to hypoplasia of erythroid elements in the bone marrow secondary to inadequate production of erythropoietin by the kidneys. Shortened erythrocyte life span, erythropoietic inhibitor substances in plasma, chronic gastrointestinal blood loss, nutritional abnormalities (e.g., iron deficiency) and bone marrow fibrosis may further exacerbate anemia in some patient with CKD [1, 13]. Hormone replacement therapy using human recombinant erythropoietin (rHuEPO) has been shown to be effective in correcting anemia of CKD dog [14]. Compared with regular rHuEPO, darbepoietin has a longer half-life and greater potency enabling the same clinical efficacy with less frequent administration [15]. In this case, the hemoglobin concentration was maintained within 13 g/dL with monthly maintenance therapy of Dabepoietin Alfa.
Uremic crisis and clinical signs should be corrected before instituting the general principles of medical supportive treatment. The advanced effective therapies for CKD dogs include peritoneal dialysis, hemodialysis, and kidney transplantation [1]. However, these effective therapies require specialized novel facilities and experts.
In the recent years, the efforts have been undertaken to mitigate uremia in animals and humans by administration of live cultures of naturally existing or genetically engineered microbes [8]. In terms of nutrition, probiotic bacteria are commonly defined as live microorganisms, which, when administered in adequate amounts, confer a health benefit to the host [8, 9]. Among toxicants accumulated in the body of dogs and cats with CKD, urea is the predominant nitrogen waste by product of protein catabolism. Although mechanisms involved in urea’s toxicity are poorly understood, it is well established theory that urea contributes to the synthesis of other toxic moieties including guanidines and carbamylation products. Therefore the urea appears to parallel the severity of renal dysfunction [12]. The putative theory is that urease-producing probiotic species hydrolyze urea and maintain a concentration gradient that favors diffusion of urea from the blood to the gastrointestinal tract rumen [9, 10]. Therefore oral probiotic dietary supplement instilled into the gastrointestinal tract could help to convert accumulated nitrogenous wastes into nontoxic compounds [8, 9, 10]. For over a decade, researchers have kept pursuing which microbes (known as probiotics) have beneficial effects to decrease azotemia which accumulated in body as renal function declines when orally administered to CKD [8, 10, 11]. According to a previous report, the results of a probiotic combination on azotemia in cats, indicate a decrease in creatinine levels in six out of seven patients treated (86%). This study suggests that probiotic therapy is safe and effective and indicates a place for such products in management of renal failure in cats [9].
In this case report, Bene-BacPowder® was used for reduced BUN, BUN/creatinine level was evaluated and consequently the BCS evaluated here was improved in the dog with CKD. Because of BUN level was affected according to use of Bene-Bac Power® medication, It is thought that potential application in CKD therapy as a dietary supplement in probiotic product might be feasible, resulting in decreasing concentration of nitrogen containing metabolites and helping to maintain healthy kidney function.
In conclusion, oral probiotic supplement in dogs with CKD could help to decrease azotemia and biochemical imbalance. Thus this alternative therapy is expected to delay the progress of chronic kidney disease. However, series of investigation are required to elucidate the efficacy of probiotic supplementation in dogs with CKD.







