INTRODUCTION
Sedation is highly recommended for minor operations or procedures, such as gastrointestinal endoscopy, radiologic intervention, or procedures conducted only with regional anesthesia in operation theaters. Insufficient analgesia and amnesia despite administration of a certain dose of sedatives and analgesics during the procedures, may take it stressful for both the operator and the patient. This may be because of variable nociceptive stimulation and patient discomfort. The use of sedative and/or analgesic during procedures must meet the needs of the patients and operators. The safety of the patient is a principal component of sedation. Sedation should not repress airway reflex and should protect airway patency and cardiorespiratory function. Additionally, it should have good pharmacokinetic properties in terms of onset time, offset time, and controllability to adjust the varying requirements of each patient. A certain level of amnesia is necessary to alleviate emotonal stress during procedures. In addition, patients should be receptive to the administration of sedatives and it should be readily administered.
Sedation can be achieved with intravenous sedatives or anesthetic agents, such as benzodiazepines, propofol, and, in more painful procedures, opioids. The utilization of these intravenous agents has been enhanced with more sophisticated techniques that enable a reliable method of sedation control. These include patient-controlled sedation (PCS) and patient-maintained sedation (PMS). Patient-controlled drug administration is a well-established technique from which the concept of patient-controlled analgesia (PCA) was derived. PCA has been proven to be highly safe and effective in analgesia, and has a high level of patient acceptance and satisfaction [1]. PCS refers to the application of the concept of PCA for intraoperative sedation. The PCS system, in which patients administer bolus doses of sedative agents as required, has been investigated by many researchers around the world, and has been shown to be very effective and safe for patients undergoing minor surgical procedures with regional anesthesia [2, 3]. The PMS system, which provides an automatically calculated concentration of propofol by target-controlled infusion (TCI), has also been used successfully to sedate patients undergoing minor operations by maintaining a constant blood concentration. The main concern with these systems, in which the patient has control of sedative administration, is safety with regard to the depth of sedation that can be achieved and the maintenance of cardiorespiratory function. Safety depends on the adequacy of the incremental increase in dose of the sedative drug, the usefulness of the lockout time in allowing equilibration of the drug with the effect site, and patient's ability to coordinate a successful handset activation. While there is no patient-controlled sedation (PCS) system available domestically, we have conducted a research project to develop a purpose-built PCS pump system for patients undergoing minor surgical procedures. Furthermore, we determined the precision degree of the system was determined using a precision scale.
MATERIALS AND METHODS
The purpose-built PCS system with handset was designed and manufactured to be applicable for the administration of clinically used intravenous sedatives. It is operated using the newly developed software algorithm that can adjust the doses and lockout times to meet the needs of the physicians as well as the patients. The pilot PCS pump included a conventional syringe with a piston to be pushed linearly. The pump is composed of a stepping motor with a 250 gear ratio that enables microinfusion by reducing the number of revolutions (revolutions per minute, rpm), a rotary encoder (in tendem with the stepping motor) on the screw guide to control the rpm, and a conventional motion controller (one channel. The motion controller will be replaced with a printed circuit board (PCB) in the follow-up research). Other parts of the pump include a serial port on the controller to monitor the syringe piston speed and the accumulated infusion data in real time, and a touch screen for the input of the calibration factor and monitoring of the infusion state.
As a simple modification of the pre-existing infusion pumps in widespread use, most of the previous similar PCS systems are differently set in their doses of sedative agents administered at the activation of handset by the patient and lockout times for investigational purposes. Our system was designed to titrate the doses and lockout time by simply modifying the software. To confirm the operating mechanism of the pump system through experimental verification, the number of steps for handset activation by the patient was limited to four steps and lockout time to 30 s. Of the intravenous sedative agents currently used clinically, propofol was chosen for experimental verification of the working mechanism by reflecting its blood concentration at each handset activation.
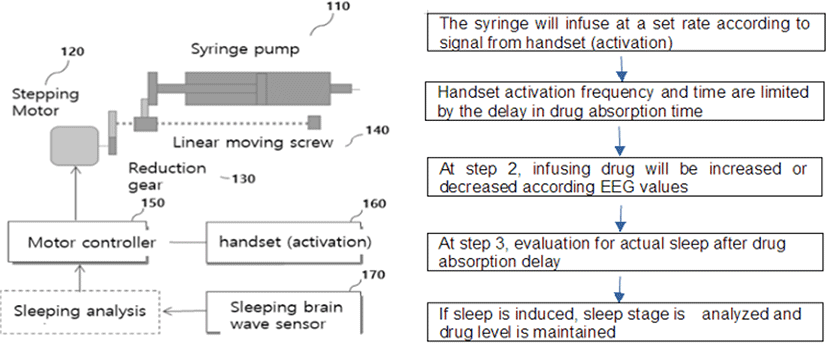
An operating algorithm for the PCS pump was developed with actual changes in blood concentration (μg/mL) according to the injected sedative (propofol) dosage (Fig. 1). The graph shows the drug mass per press (DMPP) injected with each of the handset activations and changes in the actual blood concentration fo the drug. In practice, patients are sedated by either (a) or (b). Therefore, achievement of sedation through a patient’s self-controlled handset activation is the patient’s optimal dose (i.e., 2‒3 μg/mL). We set the number of handset activations (the administraton of drug is only provided by button press of handset) in the PCS pump up to four steps for patients' safety, which would be suitable based on the drug concentraton required for the patients being sedated.
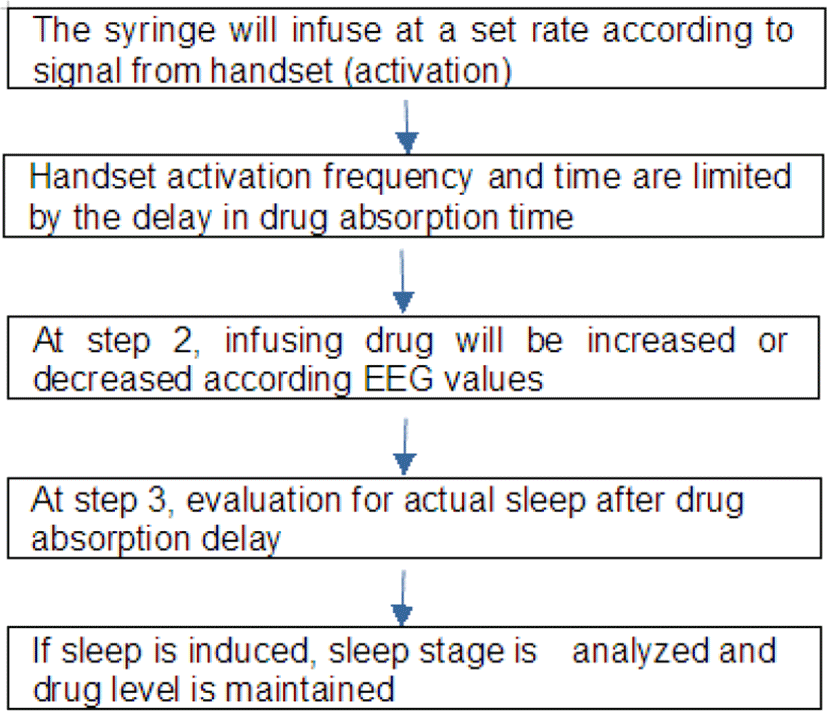
Operating instructions for the PCS pump are illustrated in flowchart (Fig. 2). The flowchart shows that the operating system runs the pump in steps. Once the patient-controlled handset activation has injected the sedative at an infusion rate of less than 0.5 μg/cc through the syringe, the infusion rate is decreased or come to halt. In the first step, the number of handset activations and time intervals are limited by the time delay between the infused dose and the actual blood concentration of the drug. After absorption of the drug, an additional third step can be followed in order to precisely control the infusion and maintain the patient’s safety by minimizing the dose infused until the patient has been induced to sleep. However, because of the delay in time to achieve the actual drug level from absorption, it is recommended that the lock-out time should be at least 30 s.
RESULTS
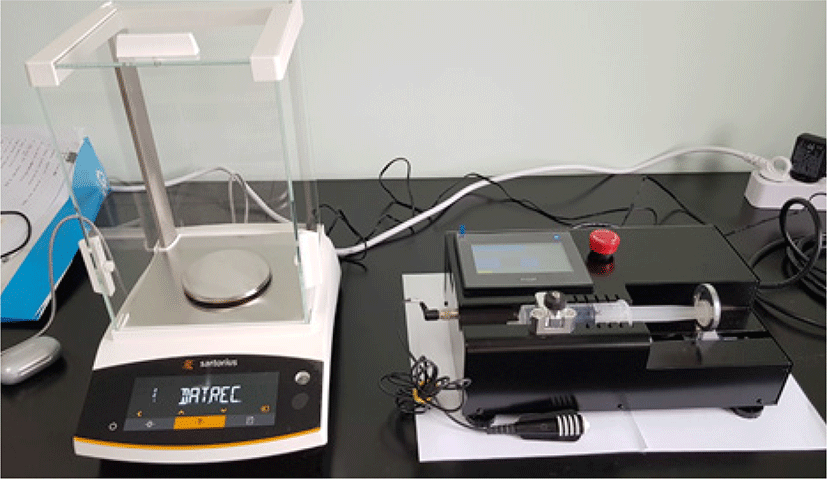
The first prototype of the manufactured PCS pump is illustrated in Fig. 3. The syringe moves in a linear direction by the force of the lead screw (pitch 0.5 mm), stepping motor (3,600 puls/rev) and motion controller. A handset was installed for the patient to press a maximum number of five times to achieve sleep, and the infusion rate per the handset activation was designed to be changed by software modification. A 4-inch touch screen monitor was installed to monitor the infusion rate as well as to control the settings that included the calibration factor, step infusion time, and maximum infusion rate.
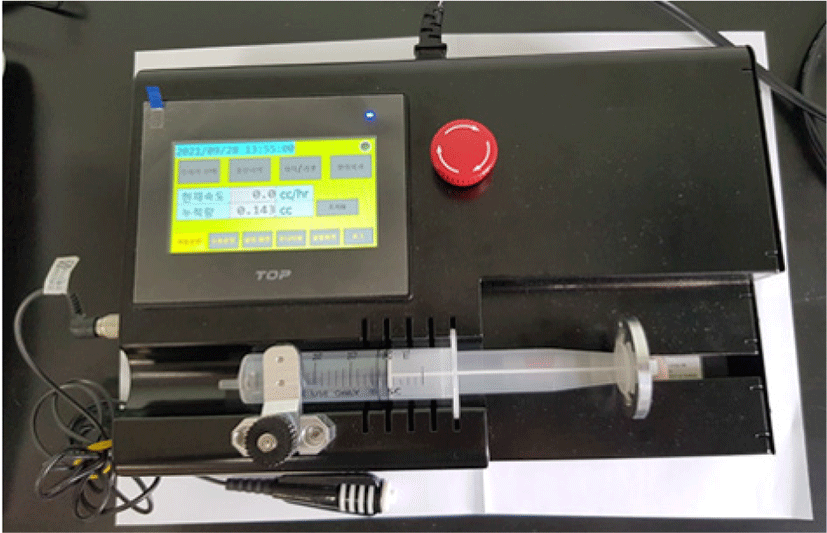
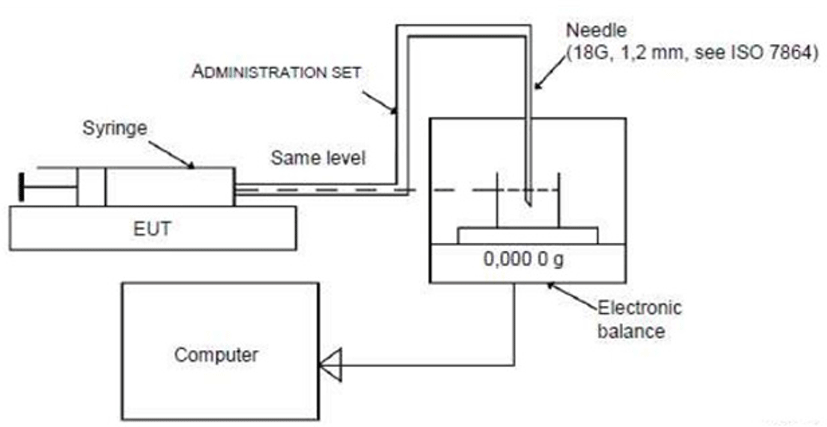
Fig. 4 shows the test method of electronic medicine infusion pump (IEC 60601-2-24:2012) using an electronic balance capable of precisely measuring the infusion rate at the same level to minimize the effect of gravity [4]. The precision test of the manufactured PCS pump was carried out using a digital weighing scale (resolution 0.1 mg) (Fig. 5). Pure water was used as the working fluid of the PCS pump instead of a sedative after calibrating at a density of 0.998 g/mL (20℃). The density of the liquid used in this experiment was corrected using water, and the outlet tube from the pump was firmly connected to the measuring dish on the scale with the tip in the water to block the airflow.
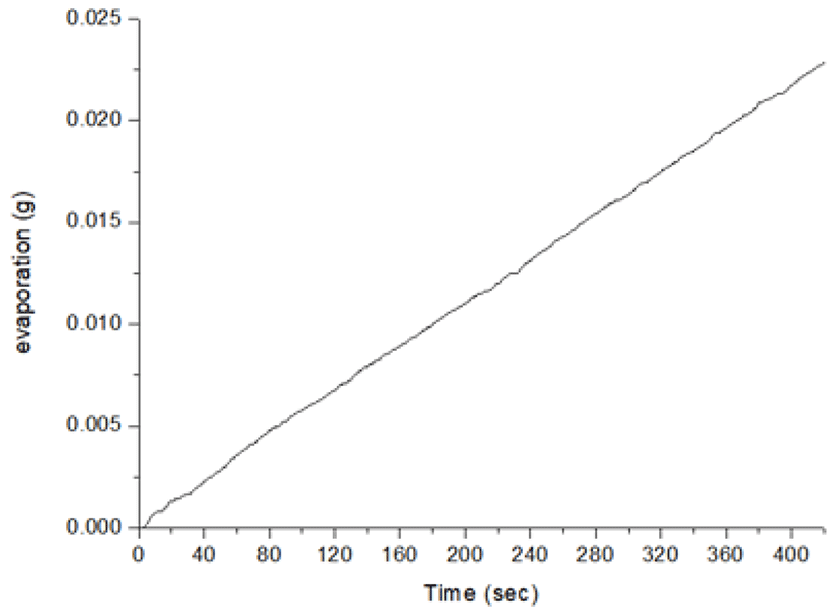
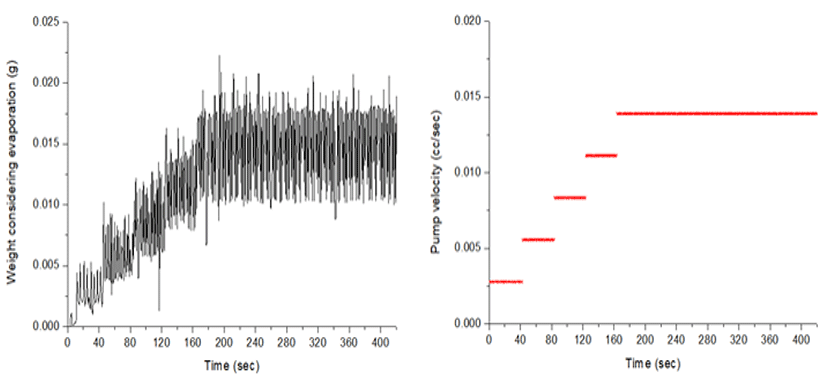
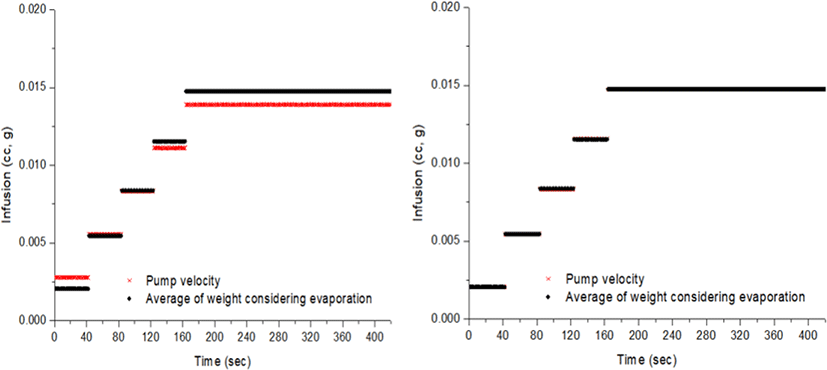
In the measurement of microweight over time, water evaporation should be considered. The evaporation loss varies depending upon the atmosphere, temperature, humidity and the type of fluid [5]. Fig. 6 shows the volume of water evaporated over a period prior to the experiment under the same conditions. The volume infused by the PCS pump was compared with precise measurements on a digital weighing scale (Fig. 7). The measured amount displayed on the scale is the volume that did not take water evaporation into consideration for five steps, with infusion increasing in 0.02 cc increments every step. As stepwise infusions are made, the command value of the piston location of the controller is shown on the PCS pump. The measured amount on the scale shows the fluctuation of the graphs a little different from the infusion steps, presumably because of the friction of the rubber piston of the syringe. A comparison between the volume infused by the PCS pump and the precise measurement using the digital weighing scale is shown in Fig. 8. The measurements on the scale are presented as mean values at each infusion step.
DISCUSSION
Clinically the ultimate goal of patient sedation is safety and effectiveness, facilitating the scheduled procedures, and PMS can help in achieving this goal. PMS is based on the TCI system, which incorporates population pharmacokinetic data describing the distribution and elimination of propofol in its microprocessor. The system calculates the initial bolus and variable infusion rates to achieve and maintain the desired concentration, and provides the amount required for effect site concentration. The handset connected to the system enables the patient to increase the target blood concentration of propofol by increasing the desired dose (μg/mL) by pressing the handset button within a second, and then a lockout interval of a few minutes is permitted during which no further increases are allowed after a maximum target concentration is reached. There are no commercially available PCS and PMS systems globally. Several purpose-built PMS systems have been used only for clinical investigations across the US and Europe. Our PCS system has been developed as an intermediate system for the ultimate PMS system, which would require further studies and developments.
Sedation for minor procesures is usually conducted by the physicians with intermittent bolus doses of propofol or midazolam regardless of the addition of opioids such as fentanyl or alfentanil. The morbidity associated with this technique has been reported to be 0.5% and fatality of 0.05% mainly due to cardiorespiratoty complications [6]. Of the reported cases of morbidity and fatality, more than 50% are related to hypoxia and deep sedation [7], mandating the use of supplemental oxygen under the standard monitoring [8]. Each of the sedative agents and opioids has its own pharmacodynamic and pharmacokinetic profiles, which influence the choice of drugs and the safety of patients, as well as the techniques of sedation.
In a study comparing propofol and midazolam for the PCS, propofol was suggested to be more suitable than midazolam in terms of sedation success rate, less impaired postoperative memory and rapid recovery, but there was no significant difference in patient satisfaction [9]. Another study compared propofol for PCS and midazolam for the clinician-controlled sedation (CCS) during colonoscopy. In this study, better patient cooperation, more sedation, faster discharge and higher colonoscopist satisfaction were achieved with PCS using propofol [10]. However, another clinical study showed that there was no significant difference between propofol-based PCS and CCS with midazolam and alfentanil during interventional neuroradiologic procesure [11]. Both groups reported similar levels of sedation, and anxiolysis, and preservation of cognitive function. Postulated reason why there was no difference between the PCS and CCS would be the addition of fentanyl to midazolam. Other clinical trials have investigated the suitability of PCS using propofol in minor or major suregries, such as extracorporeal shock wave lithotripsy, cataract surgery, submandibular fracture surgery, and dental procedures with or without local anesthesia [12-15]. In these studies PCS with propofol was shown to be a safe and effective anxiolytic technique for the patients undergoing surgical procedures. In addition, propofol dosage was lower in the PCS than in the CCS [15]. Although several drugs can be used as sedatives, limited options are available to be used in patients because of the nature of the surgical procedures as well as the pharmacologic effects of the sedatives, such as anxiety, agitaton, discomfort and pain. A possible drug combinaton, in which the level of sedation and cooperation were similar between the PCS and CCS, with better pain relief and higher satisfaction of clinicians in the CCS, is propofol and alfentanil for PCS and diazepam and pethidine for CCS [16]. In spite of these conflicting results on pain perception and satisfaction, PCS with propofol and alfentanil has been accepted as a safe and satisfactory technique by the patients. Safe sedation and acceptability of the PCS with propofol and alfentanil were also verified in a pilot study during colonoscopy [17]. A combination of propofol and remifentanil is a popular regimen of PCS for more painful procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) in the aspects of analgo-sedation. In a randomized, controlled trial, PCS with propofol and remifentanil was compared to CCS sedation with propofol [18]. PCS with propofol and remifentanil was a proper and well-accepted analgo-sedation technique for ERCP. CCS (anesthesiologist-managed) propofol sedation with constant propofol infusion was related to unnecessary deep sedation irrespective of the degree of patient or endoscopist satisfaction. This drug combinaton was also suggested to be superior than midazolam and fentanyl when used as PCS in terms of safety and the availability of facility [19]. The patients with propofol and remifentanil were sedated and recovered significantly more rapidly than the patients with midazolam and fentanyl. Some patients developed arterial desaturaton in the patients with midazolam and fentanyl.
In the present research, we designed and manufactured a pilot handset-type PCS pump capable of use by the patient himself for optimal sedation, and evaluated the infusion of drug by handset activation according to a preset infusion rate, confirming the proper working mechanism of the pump. The precision of the manufactured PCS pump was evaluated using the electronic medicine injection pump test method (IEC 60601-2-24:2012), with water as the liquid subject. The margin of error for the infusion accuracy of the PCS pump at each infusion step was within 3% on an average, while considering water evaporation during the test. Fluctuation of the infused liquid from the pump was observed, but it was thought to be due to friction of the piston, and was not considered to be a problem in comparison to the duration of anesthetic drug absorption in surgical procedures. Because of the delay in time of the actual drug level to absorption, it is recommended that the lock-out time should be at least 30 s. This handset-type PCS pump will be applied to patients for clinical trials upon completion of animal experiments and propofol will be used as a sedative agent in order to compare its pharmacokinetics with currently used TCI pumps. Furthermore, its safety and stability will be confirmed through a follow-up study.
Since the first introduction of the concept of PCA nearly 40 years ago, this technique has been applied to PCS which has been shown to be safer and more effective than intermittent intravenous injection of sedative agents requiring the interpretation of the patient's response to the administered drug. Given the standard of the PCS pump developed in this project, there is a growing need for the pump to be advanced to a higher level through subsequent research to administer optimal dose of sedatives to the patients during surgical procedures. As mentioned previously, to meet this requirement the concept of the TCI can be taken into account for the PMS, which can evolve into a more elaborate technique called closed loop control of sedation where the patient’s response is evaluated by electroencephalography (sleep stage) or arterial pressure to precisely control the infusion (Fig. 9). Once the absorption of the drug has been achieved, an additional step is involved during which the patient’s electroencephalography is analyzed again to determine whether the patient has been induced to sleep. This could help maintain the minimal dose of the infused drug.