Case Report
Sudden cardiac death as a naturally-occurring ventricular hypertrophy in Macaca fascicularis
Hyeon-Gu Yeo
1,4,†
, Junghyung Park
1,†
, Hyun Kuk Kim
3,†
, Joo Myung Lee
5
, Bon-Sang Koo
1
, Jeong-Hee An
1
, Chang-Yeop Jeon
1
, You Jung An
1
, Won Seok Choi
1
, Sung-Hyun Park
1
, Jincheol Seo
1
, Jinyoung Won
1
, Keonwoo Kim
1,4
, Jiyeon Cho
1
, Yu Gyeong Kim
1
, Minji Kim
1
, Jung Bae Seong
1
, Jae-Won Huh
1
, Sung Soo Kim
3,*
, Youngjeon Lee
1,*
, Kyung Seob Lim
2,*
1National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang 28116, Korea
2Futuristic Animal Resource & Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang 28116, Korea
3Department of Internal Medicine and Cardiovascular Center, Chosun University Hospital, University of Chosun College of Medicine, Gwangju 61453, Korea
4Department of Functional Genomics, KRIBB School of Bioscience, Korea University of Science and Technology, Daejeon 34141, Korea
5Division of Cardiology, Department of Internal Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea
*Corresponding author: Sung Soo Kim, Department of Internal Medicine and Cardiovascular Center, Chosun University Hospital, University of Chosun College of Medicine, Gwangju 61453, Korea, Tel: 82-62-220-3240, E-mail:
kholywater@naver.com
*Corresponding author: Youngjeon Lee, National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang 28116, Korea, Tel: 82-43-240-6316, E-mail:
neurosci@kribb.re.kr
*Corresponding author: Kyung Seob Lim, Futuristic Animal Resource & Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang 28116, Korea, Tel: +82-43-240-6308, E-mail:
dvmlim96@kribb.re.kr
These authors equally contributed to this work.
© Research Institute of Veterinary Medicine, Chungbuk National University.
Received: Aug 30, 2021; Revised: Sep 10, 2021; Accepted: Sep 13, 2021
Published Online: Sep 30, 2021
Abstract
Naturally occurring left ventricular hyperplasia is a rare but lethal disease. There are very few reports of this cardiac disease in captive nonhuman primates. In a colony of Macaca mulatta (Rhesus monkey) at California National Primate Research Center, a large number of rhesus macaques were diagnosed by autopsy with naturally occurring left ventricular hypertrophy without obvious underlying diseases over a 22-year period. The confirmatory diagnosis of ventricular hypertrophy was based on findings of notable left ventricular concentric hypertrophy with reduced left ventricular lumen, which is very similar to human ventricular hypertrophy cases. This report discusses an 11-year-old Macaca fascicularis monkey (Cynomolgus monkey, crab-eating macaque), weighing 2.95 kg, that was presented for enrollment in a pharmacokinetic (PK) study. During the PK experiment, the monkey died following a sudden decrease in percutaneous oxygen saturation and heart rate. Gross and histological examinations of the heart were performed. On gross pathology, the left ventricular wall was thickened, and the chamber lumen was reduced. In histopathological examination using hematoxylin-eosin and Masson-trichrome stains, fibrosis and myocyte disarray were not observed, but an increased cell density, compared to the normal heart, was confirmed. The autopsy results confirmed left ventricular hyperplasia as the major cause of death.
Keywords: non-human primate; autopsy; hypertrophy, left ventricular; sudden cardiac death; pathology
INTRODUCTION
Naturally occurring hypertrophic cardiomyopathy can occur in many other species besides humans. In humans, about 50% of patients with hypertrophic cardiomyopathy have a predisposing genetic disorder, characterized by left ventricular hypertrophy unexplained by secondary causes and a nondilated left ventricle with preserved or increased ejection fraction [1]. Although rare, spontaneous occurrence of left ventricular hypertrophy has been reported in laboratory rhesus macaques (Macaca mulatta) [2].
This case is the first case report in Korea of an experimental nonhuman primate that died due to left ventricular hyperplasia.
CASE REPORT
In this Pharmacokinetic (PK) study, adult female (11-year-old) Cynomolgus monkeys (Macaca fascicularis) were used as the research object. This PK experiment using non-human primates was approved by the Korea Research Institute of Bioscience and Biotechnology Institutional Animal Care and Use Committee (Approval No. KRIBB-AEC-21105), and was reported in compliance with the The ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). The monkeys were maintained in individual indoor cages at the National Primate Research Center at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) The monkeys were fed commercial monkey chow (Teklad 2050™, Envigo, Indianapolis, IN, USA) supplemented with various fruits, and were provided water ad libitum. Animal health monitoring comprised microbiological tests for B virus, simian retrovirus (SRV), simian immunodeficiency virus (SIV), simian virus 40 (SV40), and simian T-cell lymphotropic virus (STLV) once a year. The results of these health monitoring tests were all negative.
The monkey in question was enrolled in a safety evaluation trial of a new drug developed for the treatment of Parkinson’s disease. On the procedure day, the animals were anesthetized with ketamine (5 mg/kg, Yuhan Pharmacy, Seoul, Korea) and atropine (0.04 mg/kg, Jeil Pharmacy, Daegu, Korea), and anesthesia was maintained with 1%–2% isoflurane (Hana Pharmacy, Hwaseong, Korea) in O2 at a flow rate of 2 L/minute using an anesthesia machine (Royal Medical, Seoul, Korea). Heart rate, respiration rate, saturation of percutaneous oxygen monitoring and body temperature were continuously monitored throughout the study.
The monkey was fixed after general anesthesia and the drug was administrated to the cisterna magna using an automatic injector. After 1 hour of drug injection, a sudden decrease in heart rate and saturation of percutaneous oxygen was observed on the patient monitor, and epinephrine (0.5 mg/kg, intravenous injeciton) and chest compression were performed immediately; however, the monkey did not recover and died.
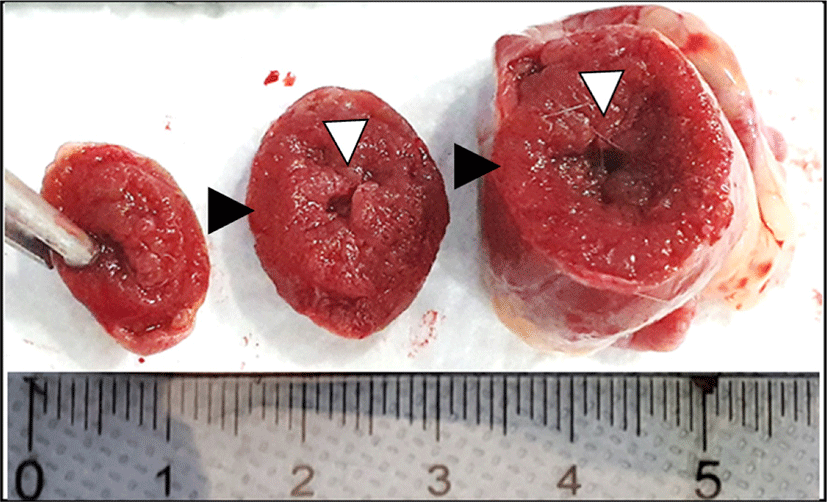
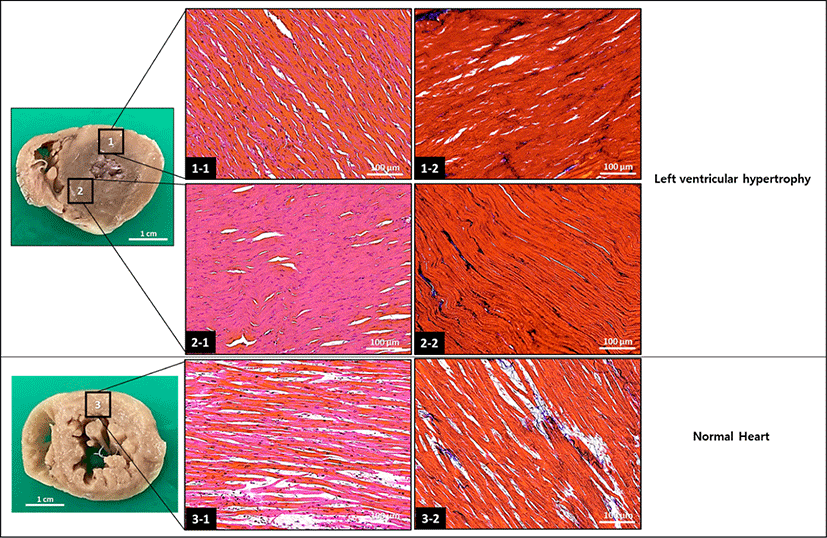
After death, an autopsy was performed, and abnormal findings were observed in the heart. In gross lesions, the ventricular wall was thickened and the lumen was reduced (Fig. 1). The extracted heart tissue was stained with hematoxylin-eosin and masson-trichrome stain. Fibrosis and myocyte disarray were not observed under histopathological examination, but it was confirmed that cell density increased compared to the normal heart (Fig. 2).
Fig. 1.
Cross sectional gross findings. A markedly increased ventricular wall thickness (black arrow head) with consequent narrowing of ventricular lumen (white arrow head) was observed.
Download Original Figure
Fig. 2.
Histopathological appearance of the formalin-fixed hypertrophic heart. The tissue sections were stained with hematoxylin-eosin (1-1, 2-1, and 3-1) and Mason-trichrome (1-2, 2-2, and 3-2). The hypertrophic heart show hypercellularity when compared with the normal heart.
Download Original Figure
DISCUSSION
Macaca fascicularis (Cynomolgus monkeys, an Old World Monkey) have been widely used as the best standard animal model in preclinical studies for new drug development because of their physiological and genetic similarity to humans [3, 4]. In Korea, Cynomolgus monkeys are commonly used as a very important and useful experimental animal for drug development. Currently, this monkey is widely used in the research of infectious diseases such as pandemic SARS-CoV-2.
In humans, heart failure is a leading cause of death worldwide [5–8]. Left ventricular hypertrophy is defined as an increase in the mass and wall thickness of the left ventricle. Left ventricular hypertrophy is the most potent reason for adverse cardiac events, and is an important risk factor for sudden death, heart failure, stroke and arrhythmia [9–11]. Therefore, left ventricular hypertrophy is commonly associated with sudden cardiac death. In studies on human patients, left ventricular hypertrophy has been found to be highly related to heredity [1, 12–15].
Except for special experimental purposes such as congenital diseases experiments, the use of healthy animals without congenital diseases is an important factor in experiments using laboratory animals. Because the monkey’s cardiac anatomy is similar to that in humans, non-human primates are a pivotal laboratory animal model in cardiovascular research, and thus, the selection of nonhuman primates with normal heart conditions is very important.
In this case, an autopsy was conducted due to the occurrence of sudden death during the pharmacokinetic experiment. This fatal disease was confirmed by autopsy in our experiment.
In cases of this condition, myocyte disarray and hypertrophy, fibrotic tissue deposition, nuclear atypia may be observed, but not necessarily [14, 16, 17]. In this case, only an increase in cell density was observed. However, left ventricular hypertrophy was confirmed by ventricular hypertrophy and reduced ventricular lumen in the heart.
Non-induced (Naturally-occuring) hypertrophic cardiomyopathy has previously been reported in a variety of species of feline, swine, and other monkey species [2, 18–20]. In humans, specific diagnostic criteria have been established, but there are no exact diagnostic criteria for Cynomolgus monkeys. We believe that it would be important to create a standard by accumulating necropsy data from cases of this disease observed monkeys.
In conclusion, echocardiography, cardiac computed tomography and/or cardiovascular magnetic resonance imaging are needed for the selection of healthy experimental non-human primates. Since hypertrophic cardiomyopathy can be inherited, particular caution is required in experimental monkeys used for breeding.
Acknowledgements
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2020R1C1C1003990) and the KRIBB (Korea Research Institute of Bioscience and Biotechnology) Research Initiative Program (KGM4562121), Korea.
REFERENCES
Olivotto I, Girolami F, Ackerman MJ, Nistri S, Bos JM, Zachara E, Ommen SR, Theis JL, Vaubel RA, Re F, Armentano C, Poggesi C, Torricelli F, Cecchi F. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc 2008;83:630-638.

Reader JR, Canfield DR, Lane JF, Kanthaswamy S, Ardeshir A, Allen AM, Tarara RP. Left ventricular hypertrophy in rhesus macaques (Macaca mulatta) at the California National Primate Research Center (1992-2014). Comp Med 2016;66:162-169.
Uno Y, Uehara S, Yamazaki H. Utility of non-human primates in drug development: comparison of non-human primate and human drug-metabolizing cytochrome P450 enzymes. Biochem Pharmacol 2016;121:1-7.


Phadke RS, Vaideeswar P, Mittal B, Deshpande J. Hypertrophic cardiomyopathy: an autopsy analysis of 14 cases. J Postgrad Med 2001;47:165-170.
Roger VL. Heart failure epidemic: it’s complicated. Circulation 2018;138:25-28.


Yamanaka S, Sakata Y, Nochioka K, Miura M, Kasahara S, Sato M, Aoyanagi H, Fujihashi T, Hayashi H, Shiroto T, Sugimura K, Takahashi J, Miyata S, Shimokawa H, Investigators C. Prognostic impacts of dynamic cardiac structural changes in heart failure patients with preserved left ventricular ejection fraction. Eur J Heart Fail 2020;22:2258-2268.



Mortazavi M. Sudden cardiac death in young athletes. Adv Pediatr 2013;60:201-215.


Calore C, De Bortoli M, Romualdi C, Lorenzon A, Angelini A, Basso C, Thiene G, Iliceto S, Rampazzo A, Melacini P. A founder MYBPC3 mutation results in HCM with a high risk of sudden death after the fourth decade of life. J Med Genet 2015;52:338-347.


Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis 2006;48:326-341.


Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, Oto A, Potpara TS, Steffel J, Marín F, de Oliveira Figueiredo MJ, de Simone G, Tzou WS, En Chiang C, Williams B. Hypertension and cardiac arrhythmias: executive summary of a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Eur Heart J Cardiovasc Pharmacother 2017;3:235-250.


Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res 2020;116:806-816.


Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol 2012;60:705-715.


Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287:1308-1320.


Ingles J, Burns C, Barratt A, Semsarian C. Application of genetic testing in hypertrophic cardiomyopathy for preclinical disease detection. Circ Cardiovasc Genet 2015;8:852-859.


Varma PK, Neema PK. Hypertrophic cardiomyopathy: part 1 - introduction, pathology and pathophysiology. Ann Card Anaesth 2014;17:118-124.


Borer JS. Left ventricular hypertrophy in hypertrophic cardiomyopathy: what’s in a phenotype? J Am Coll Cardiol 2004;44:406-408.


Huang SY, Tsou HL, Chiu YT, Shyu JJ, Wu JJ, Lin JH, Liu SK. Heritability estimate of hypertrophic cardiomyopathy in pigs (Sus scrofa domestica). Lab Anim Sci 1996;46:310-314.
Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu SK, Pion PD, Towbin JA. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation 1999;99:3172-3180.