Introduction
Cyanobacteria, also known as the blue-green algae, have been present on earth for billions of years. They have continued to evolve and adapt to the changing environmental conditions in the modern world. These cyanobacteria are responsible for the oxygenic life of the earth due to their capability of photosynthesis [1]. They are known to thrive in both marine and fresh water sources. Evolution of the world has resulted in a negative adverse effect on the natural ecological systems. Anthropogenic activities, such as industrial revolution, improper agricultural activities, indiscriminate disposal of pollutants, and the construction of reservoirs [2], trigger proliferation of these bacteria. Moreover, global warming as well as the higher nutrient content and lower flow rate of the water resource, favors growth of these bacteria. Thus, blooming of these bacteria causes a striking greenish color in the water resource. It is very common to see blooming in tropical countries, since the environmental conditions are very conducive for cyanobacterial growth in temperate zones. Blooms of cyanobacteria are most prominent during late summer and early autumn, and may last for 2–4 months. This blooming adversely affects other living beings of the water resource such as aquatic fauna and flora, as well as humans using this water for their daily requirements [3–5].
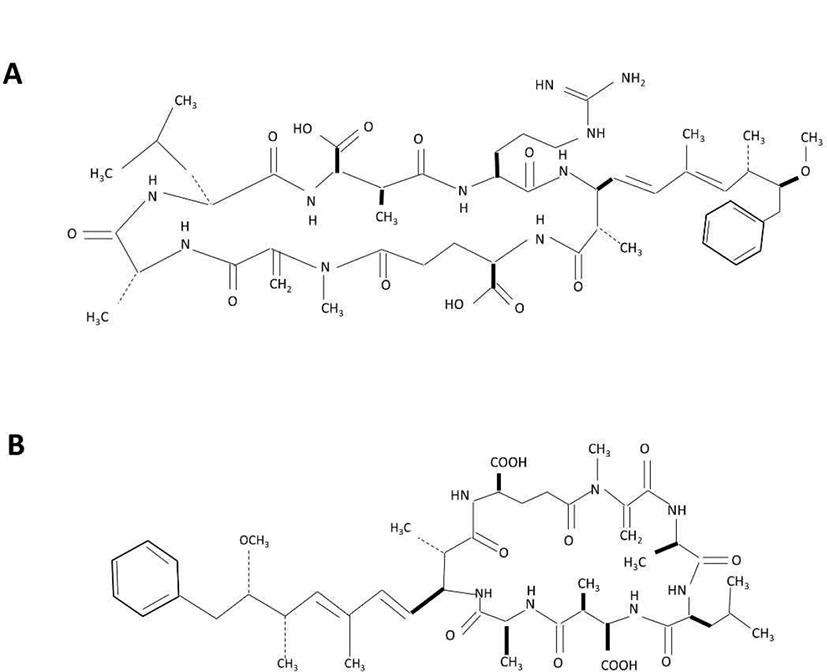
Cyanobacteria belong to the gram-negative bacterial group, producing a vast range of toxic compounds as secondary metabolites, which are mostly peptides (such as anabaenopeptins, aeruginosins, microcystins, nodularins, microginins or microviridins) and alkaloids [6]. Cyanobacteria toxin poisoning is widely reported in animals such as fish, swine, cattle, dog and bat [7]. Among the toxins produced by cyanobacteria (blue-green algae), microcystin has gained considerable attention due the ability of this substance to cause hepatotoxicity. Microcystin is a group of cyclic heptapeptide hepatotoxics that contain D- & L-amino acids, and hydrophobic amino acids [8]. The chemical structure consists of D-alanine, X, D-erythromethylaspartic acid (MeAsp), Z, 3-amino-9-methoxy-2, 6, 8,-trimethyl-10-phenyldeca-4,6 dienoic acid (Ada), D-glutamic acid, methyldehydroalanine (Mdha) [9]. X and Z indicate variable amino acids and distinguish the molecules, for instance, microcystin-LR stands for leucine (L) and arginine (R) as respective X and Z amino acid. Currently, more than 90 variants with different toxicity of microcystins have been reported. Microcystin-LR is the most well known variant, and other common variants are microcystin-LA (X: leucine, Z: alanine), microcystin-RR (X: arginine, Z: arginine), microcystin-YR (X: tyrosine, Z: arginine) [10]. Symptoms of microcystin poisoning include diarrhea, vomiting, and facial paleness; in particular, it has been reported to damage the liver cell skeleton, causing cell necrosis and bleeding [11, 12].
The evaluation of microcystin toxicity is required to predict its effect on living organisms. Planarian is an invertebrate, flat animal that is easy to breed and manage, has both asexual and sexual reproduction, and a well-developed central nervous system similar to humans; hence, it has high value as an experimental animal and is excellent for application [13, 14]. This study was therefore conducted to investigate the effect of microcystin on the motility of planarians, as well as to determine whether it is possible as an alternative experimental animal model for environmental toxin evaluation.
Materials and Methods
The planarians used in this experiment (Dugesia japonica) were propagated and maintained in water at 18°C. Planarians were fed chunks of beef liver twice per week, and water was replaced with fresh drinking water after 1 hr feeding. At least one week prior to the experiment, feeding was discontinued. Microcystin-LR (MCLR) and Microcystin-LA (MCLA) used in the experiments were purchased from Sigma-Aldrich (#33893, Seoul, Korea) and Enzo (#ALX-350-096, Madison, NY, USA), respectively (Fig. 1). Each stock solution (100 μg/mL) was prepared using methanol.
Planarian motility was measured by evaluating the swimming ability of the experimental animal in its natural state, using a petri dish (p-dish) filled with treatment solutions and divided into a grid of 0.5 cm intervals. Motility was evaluated by counting the number of grid lines that the planarians passed through [15]. Simultaneously, planarian seizure-like behaviors were also observed and the actions were classified into four categories: (A) snake-like, (B) screw-like, (C) c-like, and (D) head-bop [15]. Motility and seizure patterns were examined for 5 min, subsequent to a 5 min exposure to varying concentrations of MCLR or MCLA. Furthermore, planarians were also incubated in the absence/presence of 100 ng/mL MCLR or MCLA for 24, 48, and 96 hrs, after which they were transferred to clean water, left for 10 min to recover, with subsequent evaluation of motilities for 5 min.
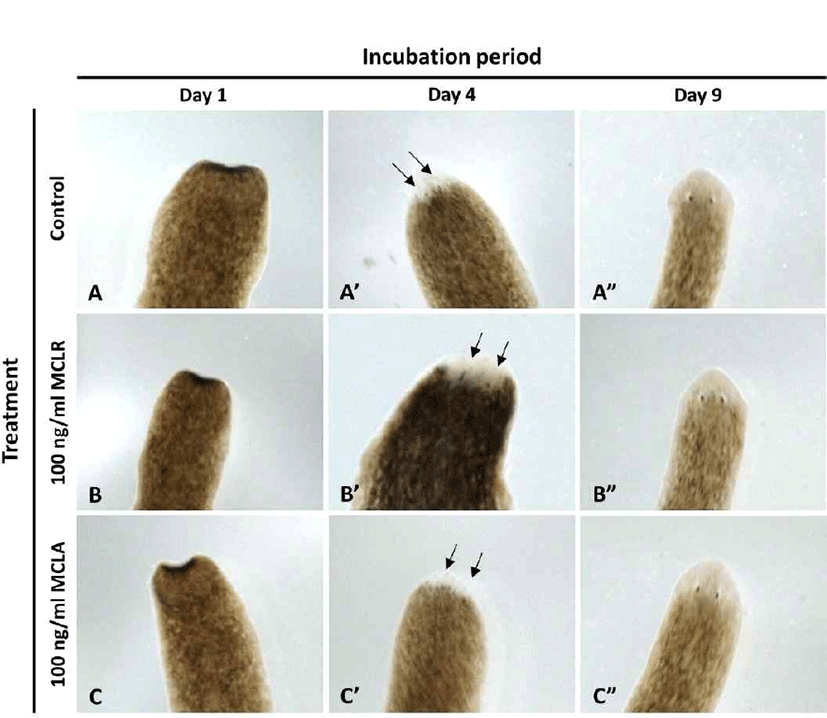
In order to examine the eye formation in planarians, the head region on the auricular grooves was cut, and rest of the body was incubated in water at 18°C until emergence of eye extrusion. The process of eye formation was observed for 9 days, under a stereomicroscope (S8APO, Leica, Seoul, Korea).
Results
Results were obtained after exposing planarians to varying concentrations of MCLR or MCLA for 5 min. Planarians were subsequently transferred to water, and motility was observed for 5 min. As presented in Fig. 2A, the number of motility decreased in planarians treated with MCLR or MCLA, as compared to controls, with no significant difference between groups. On the other hand, seizure-like behaviors were dose-dependently and significantly increased in planarians exposed to MCLR or MCLA (p<0.05, Fig. 2B). In particular, planarians treated with 10 or 100 ng/mL MCLA showed significantly higher incidence of screw type and head-bop type, as compared to other groups. Our results indicate that exposure to microcystins affects the motility and induces abnormal behaviors in planarians.
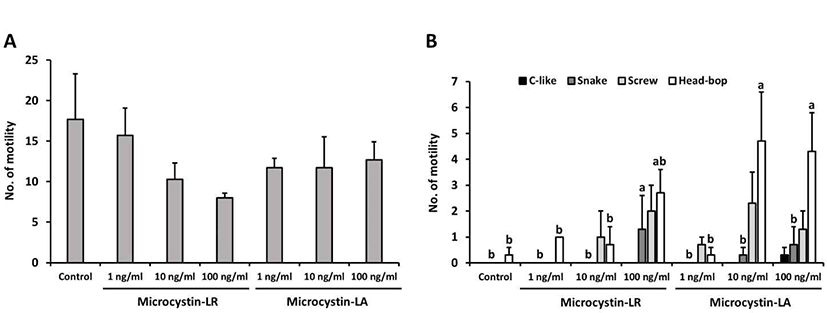
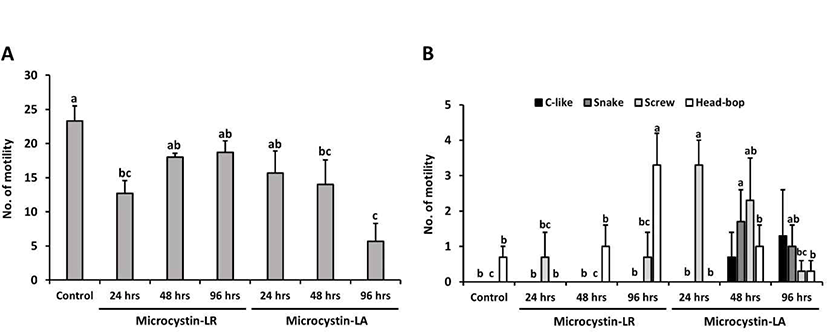
In this study, planarians were incubated in water containing 100 ng/mL MCLR or MCLA for 0–96 hrs, with subsequent examination of motility and behavior (the control group was incubated in water containing methanol for 96 hrs). In Fig. 3A, the number of motility deceased in planarians exposed to MCLR or MCLA at each exposure time; especially, MCLA-treated planarians showed significantly decreased motility with prolonged exposure (p<0.05). Moreover, the pattern of seizure-like behavior increased with increasing exposure to MCLR or MCLA, as compared to control group (Fig. 3B). The screw and head-bop characteristics were highly induced in planarians treated with MCLR for 96 hrs, whereas all seizure-like behaviors, including c-like, snake, screw and head-hop, were observed to be highly increased in planarians incubated with MCLA for 48 and 96 hrs (Fig. 3B, p<0.05). Consequently, longer exposure to microcystin resulted in increased seizure behaviors and decrease motility pattern in planarians, and showed a slightly more sensitive response to MCLA than MCLR.
In order to examine the regeneration pattern, the head region on the auricular grooves was decapitated, and rest of the body was incubated in water in the absence or presence of microcystin at 18°C, until eye extrusion was observed. As shown in Fig. 4, eye spot was observed simultaneously in all groups on day 4, and the eyes were completely formed on day 9, suggesting that microcystin does not affect eye formation, the most sensitive organ of the planarians.
Discussion
The environment is impacted most adversely with progressive revolutions in almost all fields in the world. Typically, it is the natural eco-system and organisms living in it that face maximum hardships due to the resulting pollution generated by humans. Natural water bodies are key centers of food chains and also for humans, since they provide water for the day to day activities [2, 16, 17].
Cyanobacteria, also called blue green algae, belong to the gram-negative bacterial group. They normally inhabit water reservoirs, and are engaged in photosynthesis. Pollution of water due to human activities results in increasing certain nutrient contents in the water, which ultimately leads to excessive growth, often defined as bacterial or algal blooming. Since cyanobacteria produce toxic compounds as their secondary metabolites, the increasing population of cyanobacteria results in higher toxic accumulation in the natural water resources. Consuming or living near such water bodies causes health problems to both humans and other living beings [1, 3, 4, 18]. Among the different toxic compounds, we selected microcystin to conduct the present study.
Microcystin is a substance that is continuously monitored due to concerns regarding its toxicity. In particular, controversy over the safety level of drinking water continues, and it has been designated and managed as a water quality inspection item in Korea since 2012 [19]. Microcystin can enter aquatic organisms and indirectly affect people who consume them. Moreover, exposure to low doses for prolonged periods of time leads to poisoning [20].
Planarian belongs to the group invertebrates; they have a flat body shape. Rearing them is quite easy due to their easy breeding and management requirements. They have a highly experimental value because of their well-developed central nervous system, similar to humans [13, 14]. Planarians are known to be the most sensitive to chemicals in their behavior, regeneration and brain structure, as compared to other alternative toxic animal models such as zebrafish, larvae and nematodes [21]. In addition, recent reports have suggested that planaria are useful in evaluating the toxicity of heavy metals such as copper and lead, and can be used to monitor water pollution [22].
Results of the present study reveal that motility and seizure patterns are negatively affected by both MCLR and MCLA. In some cases, the highest concentrations of microcystin showed reduction in the motility as well as increased seizure patterns in planarian (Fig. 2). Furthermore, both motility and seizure patterns are negatively affected with increasing incubation times with both MCLR and MCLA (Fig. 3). Normally, moderate or higher concentration of MCLR promotes reactive oxygen species (ROS), and subsequently results in oxidative DNA damage and transient DNA strand break [23]. The basic mechanism of this microcystin toxicity is probably the inhibition of protein phosphatases, followed by loss of cytoskeletal integrity and subsequent cytolysis or apoptosis (primarily of hepatocytes) [24, 25]. Microcystin toxicity has been reported to affect not only the liver but also the brain nerve. The neurotoxic effects by microcystin cause a variety of symptoms, namely, neuronal channels, signaling, oxidative stress, and cell skeletal disruption [26]. In this study, it was found that planarians treated with MCLR or MCLA caused more seizure-like behavior compared to motility pattern. Detailed mechanisms should be further studied in the future, but since planaria has a well-developed nervous system, it is thought that neurological seizures are more represented than motility. Previous studies provides clear evidence on the toxic biochemical reactions that occur in a living cell, which ultimately causes abnormalities in the living organism. Thus, abnormalities observed in motility, seizure and the regeneration process in the current study can possibly be attributed to alterations in the normal biochemical reactions due to the toxins.
Taken together, results of the present study indicate that there is a negative impact on the normal physical functioning of planarian, subsequent to microcystin exposure. This implies that the global increase in pollution poses a health risk to both humans and other living beings, as they have to live in an environment which is dependent on natural resources. Further studies are required to determine and express contrasting outcomes of this research area of interest.