Introduction
Foods with high calories such as sucrose and fats are rewarding and induce appetite [1]. Palatable food is often over-consumed more than needs for maintaining homeostasis and can cause obesity [2]. Although alcohol is not classified as taste stimulus, it is perceived as palatable and often over-consumed [3]. Therefore it is likely that reward system also participates in the regulation of palatable food as does taste system.
Taste information is transferred to the central nervous system from taste receptors in the oral cavity via three gustatory nerves, which are branches of facial nerves (VII), glossopharyngeal (IX), and vagus (Xth) nerves [4, 5]. The chorda tympani nerve, a branch of facial nerve innervates two thirds of the anterior tongue [5]. The first taste relay in the central taste pathway is the nucleus of the solitary tract (NST) in the medulla [6-8]. The NST sends projections to the parabrachial nuclei (PbN) in the pons [4, 6]. The PbN transmit taste information to the thalamus and ventral forebrain gustatory centers [9-11]. The lateral hypothalamus (LH), central nucleus of the amygdala (CeA), and the bed nucleus of the stria terminalis (BNST) in the ventral forebrain send efferent fibers back to the PbN and NST [12-14]. Although alcohol is not classified as taste stimulus, some gustatory neurons in the PbN and NST responded to alcohol and sucrose [15, 16].
The nucleus accumbens (NAcc) in the ventral forebrain is a pivotal neural structure in reward system and is of particular importance in drug addiction such as alcohol. Although the NAcc is not positioned in the central taste pathway, it communicates extensively with various gustatory nuclei in the ventral forebrain, including the LH, CeA, and BNST anatomically [17-19]. Investigations suggesting an important role of NAcc in mediating motivation and ingestive behavior have been reported [20-22]. For example, sucrose, high fat, or alcohol intake produces dopamine release in the NAcc [23-25]. Recently we reported that electrical stimulation of the NAcc modulates taste responses of gustatory neurons in the PbN using in vivo recording [26].
An immediate early gene c-fos, encoding the nuclear protein c-Fos, is used as a metabolic marker of brain activity [27, 28]. Sucrose sham feeding-induced c-Fos expression was observed in the NAcc as well as in other forebrain gustatory nuclei such as LH and CeA [29]. Intraventricular injection of alcohol produced c-Fos expression in various nuclei related with feeding regulation, including the PbN [30]. The purpose of the present investigation is to examine c-Fos-like immunoreactivity (cFLI) in the PbN and NAcc in response to sucrose, ethanol (EtOH), and sucrose and EtOH mixture, applied to the tongue and the relationship among them using immunocytochemistry.
Materials and Methods
The experimental procedure was approved by Animal Care and Use Committee of Gangneung-Wonju National University (GWNU2011-22). We used male Sprague-Dawley (SD) rats, weighing between 240~440 g (Orient Bio, Korea). SD rats were raised in the animal facility with free access of tap water and chow more than a week before the experiment. On the experiment day, the rat was anesthetized with intraperitoneal injection (i.p.) of Zoletil 50 (Virbac, France, 0.1 mL/100 g) and Rompun (Bayer Korea, 0.02 mL/100 g). The tongue was pulled out using forceps with dull tip, and stimulation solution-soaked cotton pad was put on it for 30 mins. The body temperature was maintained using a heating pad (Harvard apparatus, Holliston, MA, USA) during the stimulation. Stimulation solutions were 0.5 M sucrose, 10% ethanol (EtOH), and mixture of 0.5 M sucrose and 10% EtOH (sucrose/EtOH). The double-distilled water (DDW) was used as control. The data from 5 rats of each of stimulation group (sucrose, EtOH, sucrose/EtOH), and 3 rats from DDW group were analyzed.
Rat was anesthetized deeply with urethane (30%, 0.4 mL/100 g, i.p.) 1 hr after stimulation and perfused through the heart with 400 mL heparin-saline and then 4% formalin. The brain was cut into two pieces at the level of the midbrain. They were post-fixed in 4% formalin at 4°C for 2~24 hr and kept in 30% sucrose for 2~3 days at 4°C. The fixed brain was made into a cutting block using OCT compound (Frozen Section Compound, Leica, Buffalo Grove, IL, USA) and frozen at –75°C. We cut the brain in two blocks for cryosectioning considering the distance between the NAcc and PbN. Therefore, sucrose and OCT compound infiltrated thoroughly the brain more easily. The brain was sectioned at 30 μm in the coronal plane using cryostat (Cryostat, Leica, Buffalo Grove, IL, USA), and the sections were collected in 0.1 M phosphate buffered saline (PBS).
The brain sections were rinsed for 10 mins in 0.01 M PBS (pH 7.4) for three times. Three times rinsing in PBS were done between each treatment in following procedures. The sections were incubated in 0.01% NaBH4 and 0.3% H2O2 for 30 mins and then in 0.25% Triton X-100 and 2% Nomal Goat Serum (NGS) for 2 hrs. Next, the tissues were incubated in 0.25% Triton X-100 + 2% NGS + c-fos polyclonal antibody (anti c-fos Rabbit, Calbiochem, Billerica, MA, USA) overnight. The concentration of c-fos antibody solution was 1:320. Then, the tissues were incubated in 0.25% Triton X-100 + 2% NGS + secondary antibody (1:2000, Biotinylates goat anti Rabbit, Vector labs, Burlingame, CA, USA) for 2 hrs. After that, the tissues were incubated in 0.5% Triton X-100 + ABC complex (1:166, Vector labs) for 1 hr. Finally the sections were treated in 0.04% diaminobenzidine + 0.04% ammonium nickel chloride + 0.02% H2O2 for 1~2 mins and then moved in PBS for washing. The brain sections were mounted on slide glasses and dried overnight. On the following day, the slides were briefly immersed in DDW and then in neutral red solution for 2 mins. The tissue sections were dehydrated in a series of alcohol and then in xylene twice. The tissue sections were cover slipped using Permount (Fisher Scientific, Waltham, MA, USA). The reagents were purchased from Sigma (St. Louis, MO, USA), and solutions were made in 0.01 M PBS unless addressed otherwise. The procedures were done at room temperature unless addressed otherwise.
The brain sections were examined using a light microscope (Nikon, Japan). The number of cells expressing cFLI was counted using a program (NIS-Elemetns Br, Nikon, Japan) on computer connected to the microscope. Differences in number of cFLI between stimulation and DDW groups were compared using t-test or oneway ANOVA. All means were reported with SE unless addressed otherwise.
Results
We used a total of 44 rats which consisted of 11 in sucrose, 13 in EtOH and sucrose/EtOH groups, and 7 in DDW group. The NAcc is located in the ventral forebrain whereas the PbN is located in the pons of the brain stem. We examined cFLI in the PbNs and NAccs. As for the PbN sections, we tried to collect sections from the brain plane where the locus coeruleus starts to appear and to where the accessory trigeminal nucleus begins to appear. This area is relatively caudal part of the PbN, which is known to receive projections from the NST and gustatory neurons are recorded. The PbN can be classified into medial and lateral PbN. We counted number of cells with cFLI in the medial PbN because gustatory neurons are located mostly in the medial part. Fig. 1A shows the atlas brain section of the PbN, and photos in Fig. 2 demonstrate cFLI in the PbNs. The medial PbN is located medial and ventral to superior cerebellar peduncle and dorsal and lateral to mesencepahlic trigeminal nucleus.
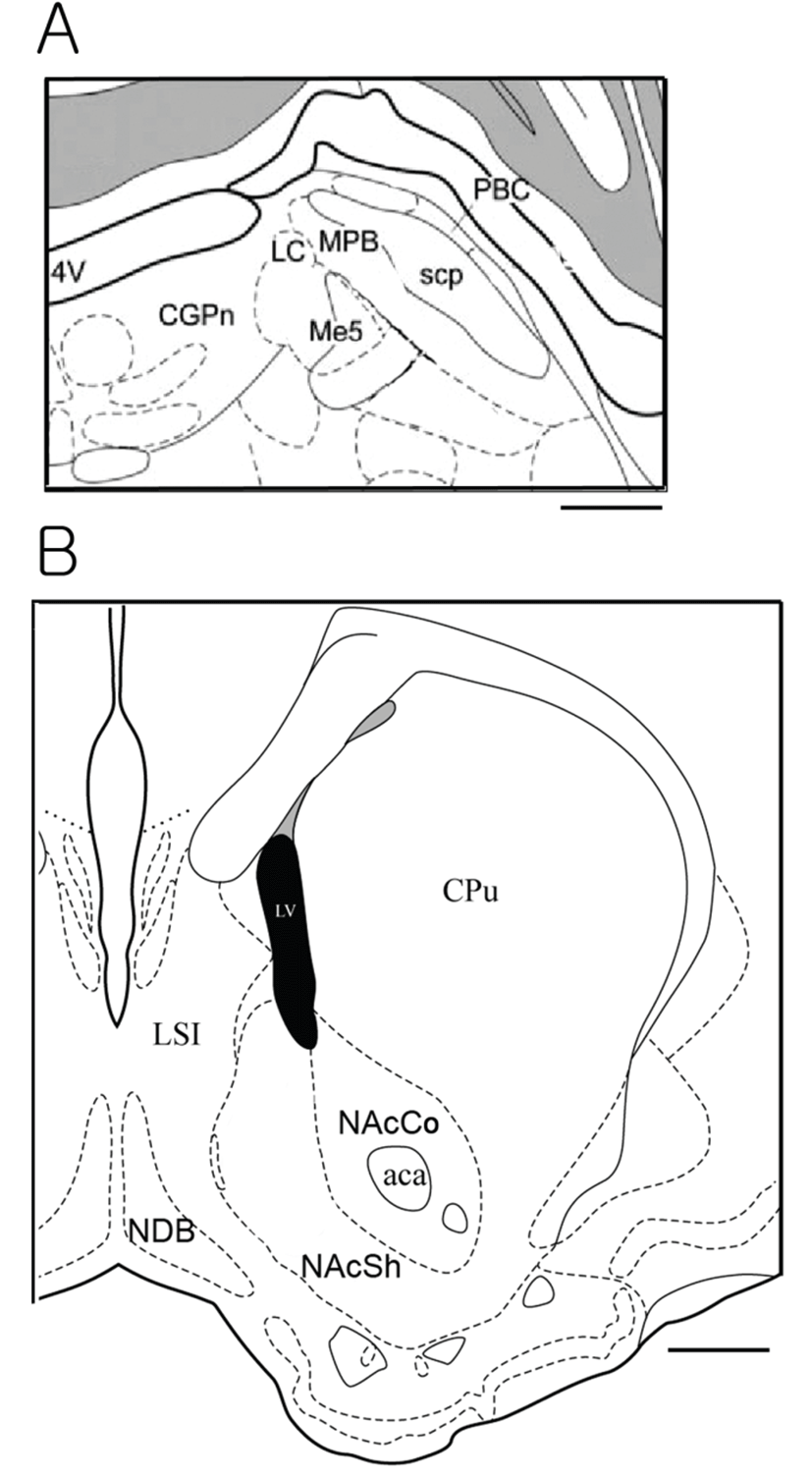

The NAcc can be classified into core and shell region.
We counted cFLI in the shell region of the NAcc because the shell area is associated with ingestive behavior. The shell region of NAcc is medial to anterior commissure and outer of the core region. Fig. 1B shows the atlas brain section of the NAcc, and Fig. 3 illustrates immunostained tissues of the NAcc. We collected tissues from the section where the anterior commissure starts to locate lateral to the lateral ventricle, until it is located in the lateral margin of the core region of the NAcc. Although 44 SD rats were experimented, some brain block was trimmed out of the target areas, and some tissues were damaged during the immunostaining. Thus, the analysis was done with the data from 18 animals (6~10 week-olds and mean weight is 431.50 ± 12.22 g), 5 animals for east stimulation group and 3 for DDW group.
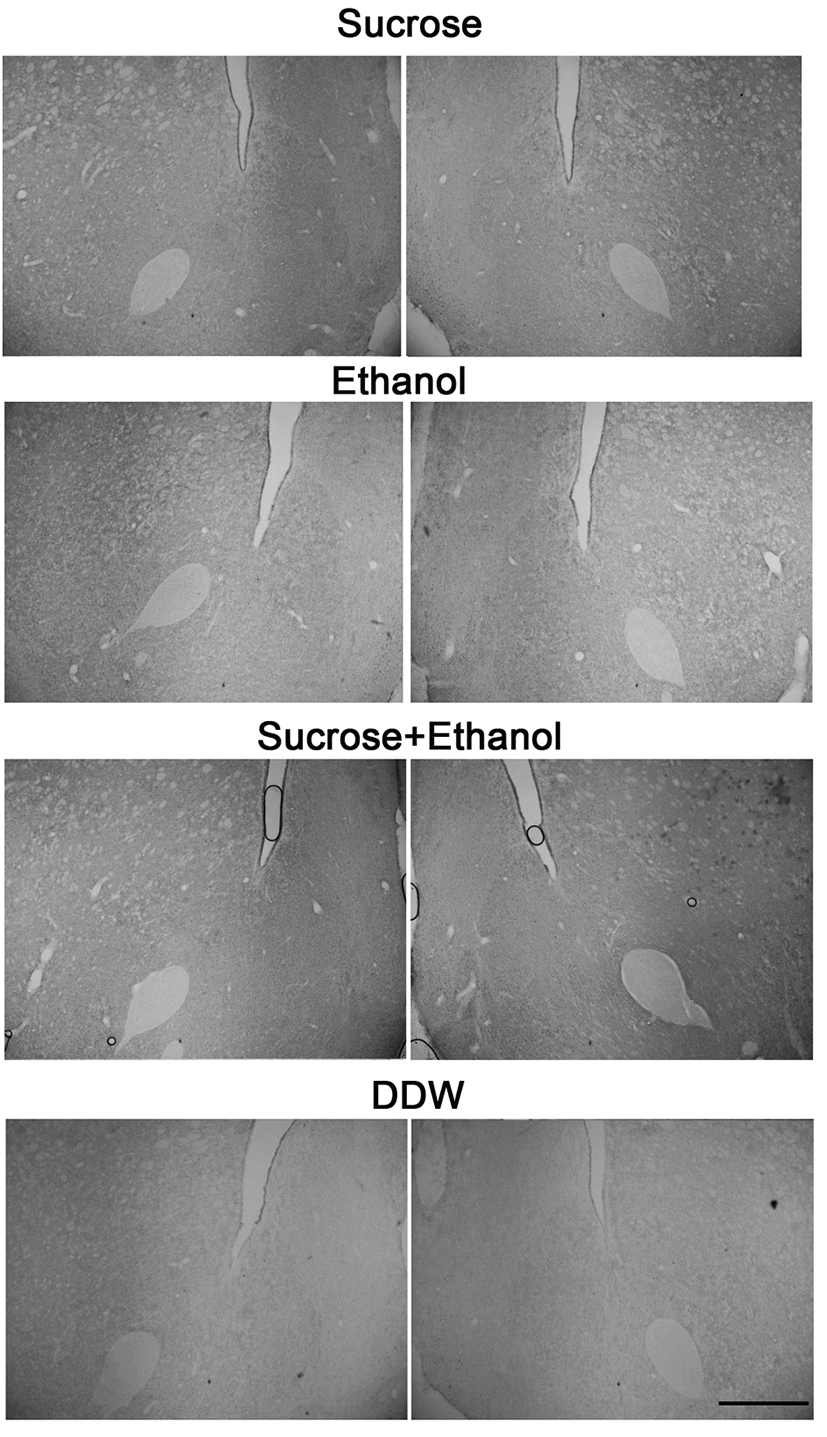
We compared cFLI among sucrose, EtOH, and sucrose/EtOH groups with cFLI in DDW groups in the PbN and NAcc, respectively. We did not distinguish the left and right of the tongue when stimulated. However, we marked the brain sections by the left and right. We compared the number of cFLI in the left and right PbNs by ANOVA. The difference between the both was not statistically significant (F[1, 24]=0.005, P=0.946). The difference between left and right NAcc was also insignificant (F[1, 24]=0.022, P=0.885). Therefore, we included the number of cFLI in the left and right PbN and NAcc for analysis.
Table 1 shows the mean (± S.E.) number of cFLI in each group. The number in the parenthesis indicates the number of cFLI of stimulation group minus that of DDW group. In the PbN, cFLI in sucrose group was significantly greater than that in DDW group (t=4.685, df=14, P<0.01). The number of cFLI in EtOH group (t=2.850, df=14, P<0.05) and sucrose/EtOH group was also greater than that of DDW group (t=2.831, df=14, P< 0.05). However, the comparison among three stimulation groups was not statistically significant (F[2, 29]=0.004, P=0.996). In the NAcc, the difference in the number of cFLI in stimulation groups and DDW group and among stimulation groups was not statistically significant (F[2, 24]=1.198, P=0.319).
Discussion
We compared the number of cFLI among sucrose, EtOH, and sucrose/EtOH groups with the number of cFLI in DDW group in the PbN and NAcc in the present study. The stimulation was applied to the anterior tongue. The access of the posterior tongue was difficult and most previous in vivo recording data were also obtained in response to the anterior tongue stimulation [31, 32]. Taste information from the anterior tongue is transmitted by chorda tympani nerve to the NST which is the first taste nucleus of the central taste pathway [4]. Majority of taste-responsive neurons in the NST send projections to the PbN, which transfers taste information to the rostral brain in two tracts [4, 6]. In addition to thalamocortical tract which transmits taste information to the gustatory cortex, the PbN projects to various nuclei in the ventral forebrain, such as LH and CeA [4, 8, 33]. The LH and CeA are related to ingestive behavior and well connected with the NAcc, which is reported to participate in regulation of feeding behavior as well [17-19]. For example, sucrose induced cFLI in the NAcc, LH, and CeA [29] or increased dopamine release in the NAcc [34].Previously in vivo electrophysiological studies reported that sucrose-responsive neurons also responded to EtOH in the NST and PbN [15, 16]. Considering the results of these investigations, we hypothesized that there would be some overlapping of sucrose-responsive and EtOH-responsive neurons in the PbN and possibly in the NAcc. If the same neurons respond to sucrose and EtOH, the number of cFLI in response to sucrose/EtOH will be less than the sum of the number of sucrose-induced and EtOH-induced cFLI. To test this hypothesis, we compared cFLIs in response to sucrose and/or EtOH application in the present experiment.
The result of the present study did not clearly demonstrate whether the same neuronal population was activated by sucrose and EtOH. The number of cFLI in response to sucrose, EtOH, and sucrose/EtOH was not different from each other. It might suggest that the same pontine neurons responded to sucrose and EtOH. However, it is not likely because some, but not all, sucrose-responsive pontine neurons responded to EtOH in the previous study [15]. In addition, stimulation-induced cFLI in the NAcc was not different from DDW-induced cFLI. We applied stimulation solution by putting stimulation-soaked cotton on the tongue of the anesthetized animal in the present study. Although most of c-Fos expression following oral stimulation of taste solutions were examined in free moving animals [35, 36], Cartens and colleagues reported that the application of EtOH to the tongue induced c-Fos expression in the brainstem [37]. Further study, in which intra-oral injection of stimuli is applied to live animal to induce c-Fos expression, may prove the hypothesis of the present study.