INTRODUCTION
Exposure to chemicals in the workplace can cause various respiratory diseases in workers. Occupational asthma is a work-related pulmonary disease caused by irritating or sensitizing factors such as dust, chemicals, or pre-existing asthmatic conditions in workers. It causes shortness of breath, tightness of the chest, and distinctive wheezing sounds [1]. Low-molecular-weight chemicals are among the various causative substances, and they include reactive compounds such as isocyanates and acid anhydrides commonly used in various manufacturing processes (e.g., production of polyurethane-based materials, including foams, adhesives, and coatings). Because of their particularly high volatility and reactivity, isocyanates such as toluene diisocyanates (TDI) or methylene diphenyl diisocyanate (MDI) can cause a significant risk of respiratory sensitization via inhalation [2]. Additionally, continuous exposure to low concentrations of air isocyanates has been associated with asthma symptoms in workers [3].
Animal testing has been a standard method for evaluating the efficacy and toxicity of new drugs as well as for assessing the safety of chemicals for several decades. However, the increased awareness of ethical issues, and cost-effectiveness of laboratory animals, and scientific limitations with regard to interspecies discrepancies in various physiological aspects have accelerated the development of alternative approaches [4]. Various animal models, including rodents, have been established for the study of asthma, and research on in vitro models has been conducted [5]. The currently available in vitro asthma models have been developed based on human lung epithelial cells [6, 7]. In this study, we used commercially available airway epithelial tissue models to evaluate the effects of known occupational asthma-inducing agents in 3D in vitro respiratory models.
MATERIALS AND METHODS
MucilAir™, SmallAir™, and SmallAir-asthma™ epithelium was purchased from Epithelix (Geneva, Switzerland) to replicate the direct structure and functional characteristics of the normal or asthmatic condition on upper or lower respiratory epithelium. The MucilAir™, SmallAir™, and SmallAir-asthma™ tissues were kept in 24-well culture plates containing 700 µL pre-equilibrated medium (Epithelix). The tissues were maintained at 37℃ in a humidified incubator with 5% CO2, and the culture medium was replenished every 2 days for 3 to 7 days.
The test materials were selected based on a list of low-molecular-weight occupational asthma-inducing agents identified in an institutional study [8, 9]. MDI (CAS number 101-68-8), TDI (CAS number 26471-62-5), and trimellitic anhydride (TMA; CAS number 552-30-7) were investigated. MDI, TDI, and TMA were purchased from Sigma-Aldrich (256439; St. Louis, MO, USA), Merck (8.08264.0100; Darmstadt, Germany), and TCI (C0046; Tokyo, Japan), respectively.
The test concentrations or incubation times for each compound were determined with reference to literature-reported IC₅₀ values [10–12]. Based on these values, the low, medium, and high concentration ranges for MucilAir™ or SmallAir™ were identified, and each compound was initially solubilized in 0.2% dimethyl sulfoxide with cell culture medium considered non-cytotoxic and was then applied to the apical surface of the tissue models. An additional stability analysis for the test substance was not conducted during the exposure period. The test concentration for SmallAir-asthma™ was adjusted based on doses in the SmallAir™ model, previously evaluated and found not to exhibit histopathological features, with the MDI dose reduced and the TMA dose increased accordingly (Table 1).
After 24 hours of incubation with the chemicals, samples collected in basolateral and apical medium and were aliquoted and used immediately or stored at –80℃ until use. Cytotoxicity was determined by measuring lactate dehydrogenase (LDH) release using a commercially available kit (Cytotoxicity Detection Kit, Roche, Basel, Switzerland) according to the manufacturer’s instructions. The frozen samples were transferred to 96-well microplates and incubated in the dark with a 100 µL reaction mixture. Absorbance was measured at 490 nm using a microplate reader (BioTek Synergy HT, BioTek, Winooski, VT, USA). Each concentration was examined using two replicates, and the results were calculated as a percentage of the maximum activity controls included in the kit.
The levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the frozen samples were determined using an OxiSelectTMIn Vitro ROS Assay Kit (STA-347, Cell Biolabs, San Diego, CA, USA) in accordance with the manufacturer’s instructions. Fluorescence was measured at 480/530 nm using a microplate reader (BioTek Synergy HT, BioTek).
Cytokine and chemokine release on the basolateral medium and apical surfaces were measured using a Luminex® platform (Luminex® 200 system, LX200-XPON3.1, Luminex Corporation, Austin, TX, USA) according to the manufacturer’s instructions. Briefly, Luminex Discovery assay kits were applied for CCL2, interleukin (IL)-1β, IL-4, IL-6, IL-8, IL-13, IL-17, MMP-1, MMP-2, or TNF-α (LXSARM-10, R&D Systems, Minnneapolis, MN, USA) or TGF-β1, -2, and -3 (RND-LTGM100/200/300, R&D Systems). All reagents included in the kits were prepared according to the manufacturer’s guidelines, and each sample was diluted to the appropriate concentration.
Tissue samples were fixed in 10% neutral-buffered formalin for 24 hr and washed with phosphate-buffered saline. The tissues were dehydrated in graded ethanol, cleared with xylene, and embedded in paraffin. Paraffin-embedded tissues were cut using a microtome (Leica, Wetzlar, Germany), and the sections were deparaffinized, hydrated, washed, and stained with hematoxylin and eosin (HE; CS700 and CS701, DAKO, Glostrup, Denmark) or periodic acid Schiff (PAS) solution (ab150680, Abcam, Cambridge, MA, USA). The stained sections were coverslipped with mounting medium (CS703, DAKO) and observed under a light microscope (DM3000LED, Leica) at 20 × magnification.
Statistical analyses were performed using SPSS software (IBM, Armonk, NY, USA). One-way ANOVA or Student’s t-test was used for analysis, and Dunnett’s test or Tukey HSD test were used for multiple comparisons to determine differences from the control or Low/Middle. Differences were considered significant at p<0.05 or p<0.01.
RESULTS
The levels of LDH activity induced by each compound were evaluated in each of the 3D-cell models. Concentration-dependent increases in LDH were observed following MDI and TDI exposure in MucilAir™, whereas no remarkable change was detected after TMA exposure. In the SmallAir™, exposure to MDI and TDI led to increased LDH levels at the middle- and high-concentrations, respectively. However, high-concentration exposure of SmallAir™ to MDI and of MucilAir™ to TMA resulted in LDH levels beyond the measurement range. In the SmallAir-asthma™ model, LDH levels increased in a concentration-dependent manner after treatment with MDI and TMA, and an increase was also identified at the high concentration following exposure to TDI (Table 2).
To assess oxidative stress in 3D-cells induced by chemicals, ROS levels were measured. However, the differences were not statistically significant (Table 3).
To assess immunological factors related with asthma affected by chemical treatment in a 3D-cell model, cytokines, chemokines, and other airway remodeling markers including MCP-1, IL-1β, IL-13, IL-17, IL-4, IL-6, IL-8, MMP-1, MMP-2, TNF-α, and TNF-β1, -2, and -3 were measured. In the SmallAir™ model, MDI exposure led to a concentration-dependent decrease in IL-6, whereas TDI exposure induced concentration-dependent decreases in IL-1β and IL-8 in the SmallAir-asthma™ model. However, these alterations should be interpreted within the context of cytotoxic effects elicited by high-concentration exposure. In addition, exposure to TDI at middle and high concentrations induced a significant, concentration-dependent reduction of IL-17, IL-4, and TNF-α in the SmallAir-asthma™ model. However, the other factors exhibited no characteristic alterations in the marker profile (Supplementary Tables S1, S2, and S3).
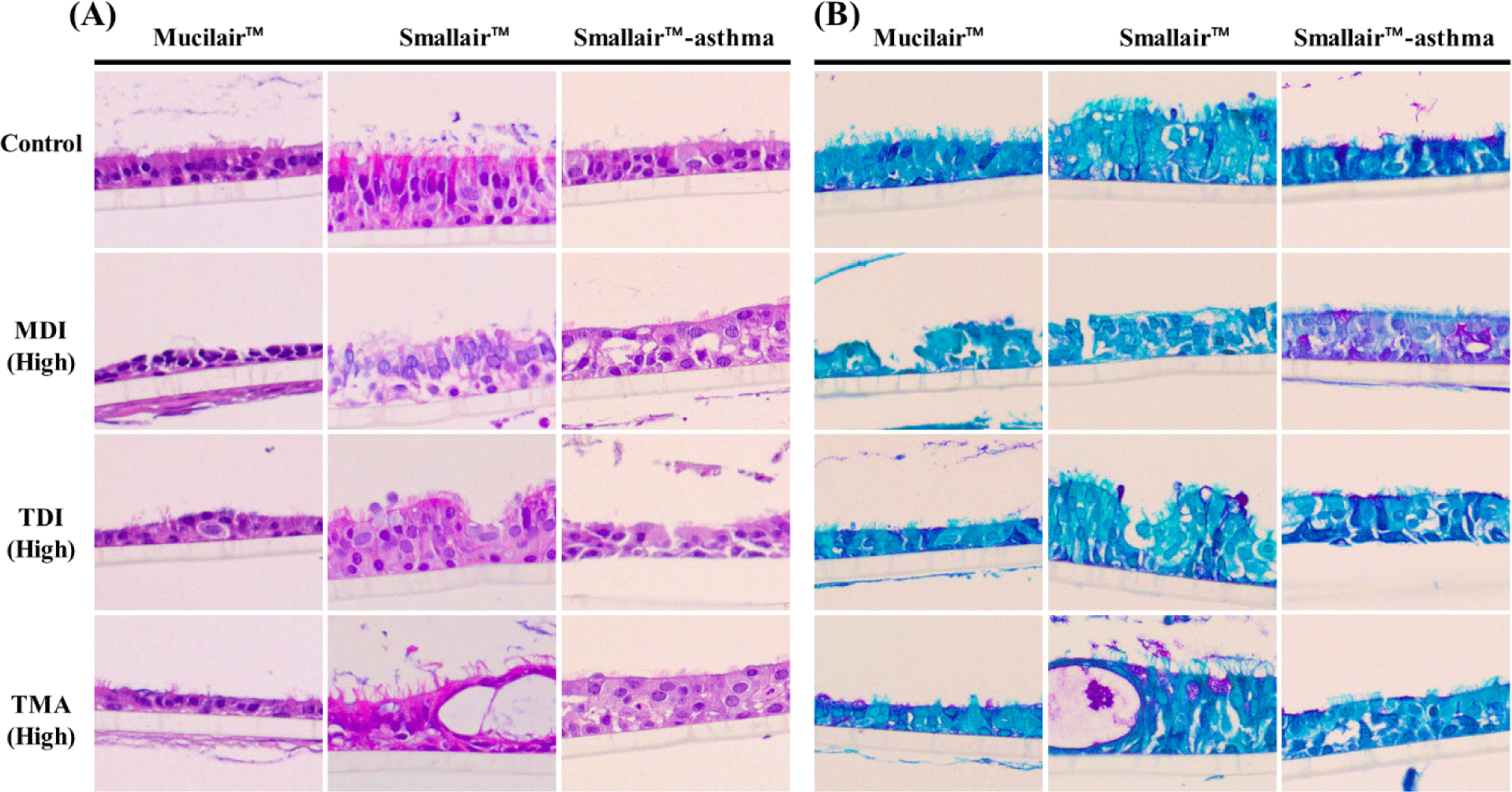
Treatment with MDI, TDI, and TMA was confirmed to damage the respiratory epithelium of MucilAir, SmallAir, and SmallAir-asthma. The non-treated 3D-models showed a well-constructed normal cell layer or a modified respiratory epithelium due to the asthmatic conditions. As a result of the treatment of the MDI and TMA, the MucilAir™ model showed changes in the height of the epithelial cells, focal epithelial loss, and disorganization of the epithelium. In addition, the respiratory epithelial cells showed pronounced cellular blebbing following TDI treatment. In SmallAir™, epithelial hyperplasia and cyst formation were observed. Degeneration of the respiratory epithelium increased in SmallAir-asthma™ (Fig. 1A). Furthermore, PAS staining revealed the presence of mucus in the epithelium (Fig. 1B). The mucus secretion induced by MDI, TDI, and TMA was not markedly increased in MucilAir™ and SmallAir™, but a substantial increase in mucus secretion was observed in SmallAir-asthma™ compared with the other models, and MDI treatment exacerbated this outcome.
DISCUSSION
This study was designed to assess the effects of chemicals known to be associated with occupational asthma in the upper and lower airways using 3D models from normal or asthmatic donors. Considering the differences in cellular composition between the upper and lower airways, MucilAir™ is composed of differentiated ciliated epithelial cells, goblet cells, and basal cells, effectively reproducing the tight junctions, polarity, and pseudostratified epithelium of the human bronchial lining. SmallAir™ is a reconstructed cell line designed for studies of the human lower respiratory tract, and unlike MucilAir™, it includes club cells. Additionally, SmallAir-asthma™ is a model created using respiratory cells from asthma patients, constructed in the same manner as SmallAir™ and used to identify differences in biological responses between normal human cell models and asthma-derived models when exposed to the same substances. These models were validated for the immune response by viruses originating from differentiated nasal epithelial cells, which secrete cytokines and chemokines, produce mucus, and remove mucus, and showed equivalent results when assessing antiviral agents using air-liquid interface (ALI)-cultured epithelial models [13, 14].
TDI is well recognized as a highly toxic chemical and has been epidemiologically linked to occupational asthma through acute exposure to high concentrations. In addition, epidemiological studies on occupational respiratory diseases have indicated that MDI is associated with both asthma and reduced pulmonary function, similar to TDI [15]. TMA is a prototypical target of occupational immune- surveillance programs in the USA and can induce a spectrum of occupational respiratory diseases, including occupational rhinitis and asthma [16].
In our study, 3D cell models exposed to MDI and TDI showed concentration-dependent LDH activity, which is an indicator of cellular damage. However, TMA exposure did not show concentration-dependent effects in any model except SmallAir-asthma™, and LDH release following treatment with 300 μM MDI and 250 μM TMA was not detected in SmallAir™ and MucilAir™, respectively. These results likely reflected rapid and massive cytotoxicity at concentrations that prevented sustained LDH release over the incubation period [17]. In addition, several studies have reported that the asthmatic epithelium is more susceptible to external irritants, including viral infections and cigarette smoke, owing to impaired defensive mechanisms [18]. However, the low LDH levels detected in asthma patient–derived epithelium (SmallAir-asthma™) after TMA exposure were inconsistent with previous research and require further study.
Unexpectedly, no detectable alterations in ROS/RNS levels were observed, which are indicators of oxidative stress damage. ROS/RNS are critical mediators of inflammatory immune responses, functioning through signaling pathways such as NRF2, p38 MAPK, and NF-κB, and are characterized by high reactivity and short lifetime, often lasting only a few minutes [19, 20]. In the present experiment, the short half-life of ROS may have limited the ability to detect oxidative stress in 3D-cell models. It is well established that occupational asthma-related chemicals increase ROS levels and initially induce secretion of IL-6 and IL-8 processes that promote inflammation and fibrosis in vitro and in vivo [10, 21, 22]. In general, the asthmatic airway is characterized by an increased number of aberrant basal cells and goblet cell hyperplasia, together with impaired ciliated cell function; these changes are evident in HE and PAS staining of SmallAir-asthma™ tissues [18]. Furthermore, epithelial cells derived from patients with asthma have been reported to exhibit delayed injury repair in ALI-cultured airway epithelial cells and associated abnormal cellular mitosis and increased TGF-β driven extracellular matrix production [23, 24]. The reduced levels of MCP-1, IL-6, IL-8, MMP-2, TNF-α, and TNF-β1/2/3 detected in cytokine analyses of SmallAir-asthma™ samples may be related to these abnormalities when compared with SmallAir™ tissue from normal donors.
In the present study, we examined asthma-related factors, including cytokines and chemokines. In both the SmallAir™ and SmallAir-Asthma™ models, exposure to MDI or TDI was associated with decreases in IL-6, IL-1β, IL-8, IL-17, IL-4, and TNF-α. Although various mechanisms of asthma have been suggested, Th2 cytokines such as IL-4 and IL-13 are crucial factors in the airway epithelium and are implicated in the pathogenesis of asthma [25]. Asthma is associated with an imbalance in Th2 and Th1 responses in immune cells [26, 27]. Huang and colleagues investigated respiratory sensitizers with a dextran carrier in MucilAir™ and identified candidate biomarkers of respiratory sensitization, such as IL-6, IL-8, Gro-α, RANTES, and MCP-1, despite differences in response time [6]. The authors proposed that epithelial NF-kB activation would be the dominant mechanism in the MucilAir™ model; however, experimental validation is still lacking. Further, NF-κB signaling played key roles in respiratory inflammation, infection, and ROS-associated processes, and it was suggested that the respiratory sensitizers activated NF-κB aberrantly and persistently in airway epithelial cells to drive overexpression of Th2 cytokines [6]. By contrast, recent studies reported that NF-κB is associated with respiratory inflammation but is not a major regulator of Th2 differentiation and may be more closely linked to Th1 responses, suggesting that asthma pathogenesis involves multiple immune axes and that co-culture models including Th1, Th2, Th17, and other immune populations are required for further investigation [28, 29].
The ALI-based 3D-cell models used in the present study have limitations for studying airway remodeling and metaplasia, but they are suitable for investigating epithelial-derived cytokines and related factors [30]. In summary, we evaluated the effects of low-molecular-weight occupational asthma–associated compounds, including MDI, TDI, and TMA, on several 3D-cell models. Epithelial damage was indicated by LDH release and histological features, whereas oxidative stress markers or changes in canonical cytokine levels were not observed. These findings were likely due to the rapid hydrolytic reactions of the highly electrophilic test compounds (MDI, TDI, and TMA) with water [6]. Further studies are needed to establish whether the 3D-cell models can demonstrate a variety of immune responses induced by asthma-related chemicals.