INTRODUCTION
High-mobility group box-1 and 2 (HMGB1 and HMGB2) are non-histone nuclear proteins belonging to the HMG superfamily that help regulate various genomic operations, including DNA repair, transcription, and nucleosome sliding [1–3]. These two proteins are highly expressed in all mammalian tissues during embryogenesis. In specific tissues, they have been found both inside and outside the nucleus, with the testis and ovary displaying higher levels in the nucleus and cytoplasm [4–6]. HMGB1 initiates inflammatory responses when it interacts with cell surface receptors, such as the receptor for advanced glycation end products (RAGE) and toll-like receptor 4 (TLR4), to influence the levels of proinflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α [7, 8]. Extracellular HMGB2 promotes the proliferation and migration of endothelial cells and influences these functions by engaging RAGE [9]. The porcine species serves as a distinctive model for conducting research in the field of human developmental biology and assisted reproductive technology, such as in vitro fertilization (IVF), and has been extensively employed in investigations pertaining to porcine reproduction and development of early-stage embryos [10]. Developing effective in vitro methods for generating porcine embryos has been challenging due to the frequent occurrence of polyspermic fertilization, which is more common in pigs than in other species [11]. Glycyrrhizin (or glycyrrhizic acid [GA]) is a triterpene glycoside, the primary constituent derived from the licorice root (Glycyrrhiza uralensis). It has been reported that GA inhibits the Ca²⁺- and phospholipid-dependent phosphotransferase activity of protein kinase C (PKC), the phorbol ester tumor promoter receptor. GA has also been found to exhibit various pharmacological effects, including anti-inflammatory, anti-ulcer, anti-allergic, anti-carcinogenic, and immunomodulatory actions [12]. In addition, GA has been demonstrated to be a natural inhibitor of extracellular HMGB1 [13, 14]. Previous studies have reported that the addition of an aqueous extract of licorice to the artificial insemination (AI) culture medium increases the IVF rates in mouse oocytes [15]. Therefore, the purpose of this study was to investigate how GA affects the fertilization and early development of porcine oocytes fertilized in vitro, specifically focusing on the potential involvement of the HMGB1 protein in the pre-implantation stage of embryo development.
MATERIALS AND METHODS
Liquid boar semen was purchased from a local AI center and stored at 17℃ for 5 days prior to use.
Ovaries were collected from prepubertal gilts at a local slaughterhouse and transported to the laboratory. Cumulus-oocyte complexes (COCs) were aspirated from the antral follicles (3–6 mm in diameter), washed three times in N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES)-buffered Tyrode lactate (TL-HEPES) medium supplemented with 0.01% (w/v) polyvinyl alcohol (PVA; TL-HEPES-PVA), followed by three washes with the oocyte maturation medium [16]. A total of 50 COCs were transferred to 500 µL of the maturation medium and layered with mineral oil in a 4-well multi-dish equilibrated at 38.5℃ and 5% CO2 in air. Tissue culture medium (TCM)199 was used as the oocyte maturation medium. It was supplemented with 0.1% PVA, 3.05 mM D-glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 0.5 µg/mL luteinizing hormone (Sigma-Aldrich, Seoul, Korea), 0.5 µg/mL follicle-stimulating hormone (Sigma-Aldrich), 10 ng/mL epidermal growth factor (Sigma-Aldrich), 75 µg/mL penicillin G, and 50 µg/mL streptomycin. The oocytes were cultured in TCM199 for 44 hr at 38.5℃ and 5% CO2 in air.
After in vitro maturation (IVM), the cumulus cells were removed by treatment with 0.1% hyaluronidase in the TL-HEPES-PVA medium, and metaphase II oocytes (MII) were selected by observation under a stereomicroscope. Thereafter, oocytes were placed into four 100 μL drops of modified Tris-buffered medium (mTBM) in a 35-mm polystyrene culture dish and covered with mineral oil. One milliliter of liquid semen stored in Beltsville Thawing Solution was washed twice with phosphate-buffered saline (PBS) containing 0.1% PVA (PBS-PVA), at 800 × g for 5 min. The washed spermatozoa were resuspended and diluted in mTBM. For IVF, 1 µL of the sperm suspension was added to the medium containing the oocytes to give a final sperm concentration of 1 × 105 spermatozoa/mL. Oocytes were co-incubated with spermatozoa for 5 hrs at 38.5°C and 5% CO2 in air. After IVF, oocytes were transferred to 500 μL porcine zygote medium (PZM-3) [17] supplemented with 0.4% bovine serum albumin (BSA, A0281, Sigma-Aldrich), and cultured for an additional 20, 48, or 144 hr. To observe the effects of GA on IVF and in vitro culture (IVC), various concentrations of GA were added into the mTBM (0–200 µM GA) or PZM (0–100 µM GA), respectively. GA (Glycyrrhizic acid ammonium salt G2137, Sigma-Aldrich) was dissolved in DMSO for use, and the addition amount was limited to no more than 0.5% (v/v) in both IVF and IVC media. The IVM, IVF, and IVC studies were repeated four times for each treatment regimen.
Oocytes/embryos were fixed with 2% formaldehyde for 40 min at room temperature (RT), washed twice with PBS, permeabilized with PBS-Triton X-100 for 30 min, and stained with 2.5 mg/mL 4′,6-diamidino-2-phenylindole (DAPI; DNA staining; Molecular Probes, Eugene, OR, USA) for 40 min. The fertilization status of the zygotes (unfertilized, fertilized-monospermic, or fertilized-polyspermic), cleaved embryo number, blastocyst formation, and the cell number per blastocyst were assessed under a fluorescence microscope (Nikon Eclipse Ci microscope; Nikon Instruments, Tokyo, Japan).
Spermatozoa, oocytes, embryos, or blastocysts were fixed with 2% formaldehyde for 40 min at RT, washed with PBS, followed by permeabilization in PBS and 0.1% Triton-X 100 (PBS-TX) for 40 min at RT. The membrane blocking was done by PBS-TX containing 5% normal goat serum for 20 min. Subsequently, the fixed cells were incubated with rabbit monoclonal anti-HMGB1 antibody (ab79823, Abcam, Cambridge, UK) or rabbit monoclonal anti-HMGB2 antibody (1:1,000 dilutions, ab124670, Abcam) for 40 min at RT. After washing with PBS-TX, the cells were incubated with goat anti-rabbit IgG Alexa Fluor™ 488 (A-11008, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) for 40 min at RT. Spermatozoa were incubated with 5 µM MitoTrackerTM Red CMXRos (Thermo Fisher Scientific) for mitochondria labeling, and the DNA was stained with DAPI. All immunofluorescence signals were visualized in a fluorescence microscope, and images were captured using a fluorescence microscope (Nikon Instruments).
Total protein was extracted from the testis samples using lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% deoxycholic phenylmethylsulfonyl fluoride, 1 µg/mL aprotinin, 5.0 Mm sodium pyrophosphate, 1 g/mL leupeptin, 0.1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol) on ice, and protein concentrations were measured using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were subsequently separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% gel and electrophoretically transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories). The membranes were blocked with 5% skim milk in tris-buffered saline containing Tween-20 for 1 hr at RT, and then incubated with rabbit monoclonal anti-HMGB1 antibody, rabbit monoclonal anti-HMGB2 antibody (1:1,000 dilutions, Abcam) at 4℃ overnight. Blots were incubated with goat anti-rabbit immunoglobulin G-horseradish peroxidase (IgG-HRP) secondary antibody (#31460, Thermo Fisher Scientific) for 1 hr at RT. The immunoreactive bands were detected by enhanced chemiluminescence detection reagents (#34096, SuperSignalTM West Femto Maximum Sensitivity Substrate, Thermo Fisher Scientific) using the Davinch-Chemi Fluoro imaging system (Davinch-K, Seoul, Korea).
Values are presented as the mean ± S.E.M. Data analyses were conducted using one-way analysis of variance (ANOVA) with the SAS package 9.4 (SAS Institute, Cary, NC, USA). A completely randomized design was used, and Duncan’s multiple range test was performed to compare the values of individual treatments when the F-value was significant (p<0.05).
RESULTS
Visualization with immunofluorescence microscopy provided a detailed view of the localization of HMGB1 (Fig. 1). HMGB1 was observed in boar spermatozoa, including the head post-acrosomal sheath and the mid-piece of the sperm tail (white arrows in Fig. 1A). Accumulation of HMGB1 could be observed in the nuclear region of immature oocytes (germinal vesicle [GV] in Fig. 1B), and it migrated into the cytoplasm of the oocyte during the mature metaphase II (MII) stage (Fig. 1C). HMGB1 was seen in the zygotic pronuclei (PN in Fig. 1D), embryonic stage (Fig. 1E), and hatched blastocyst nuclei, respectively (Fig. 1F). Particularly, in pronuclear formation stage, strong expression of HMGB1 was observed in both the pronuclei and cytoplasmic regions (Fig. 1D). Subsequently, cytoplasmic expression decreased (Fig. 1E), but its localization was again clearly identified in both the nuclei and cytoplasm of pre-implantation blastocysts (Fig. 1F).
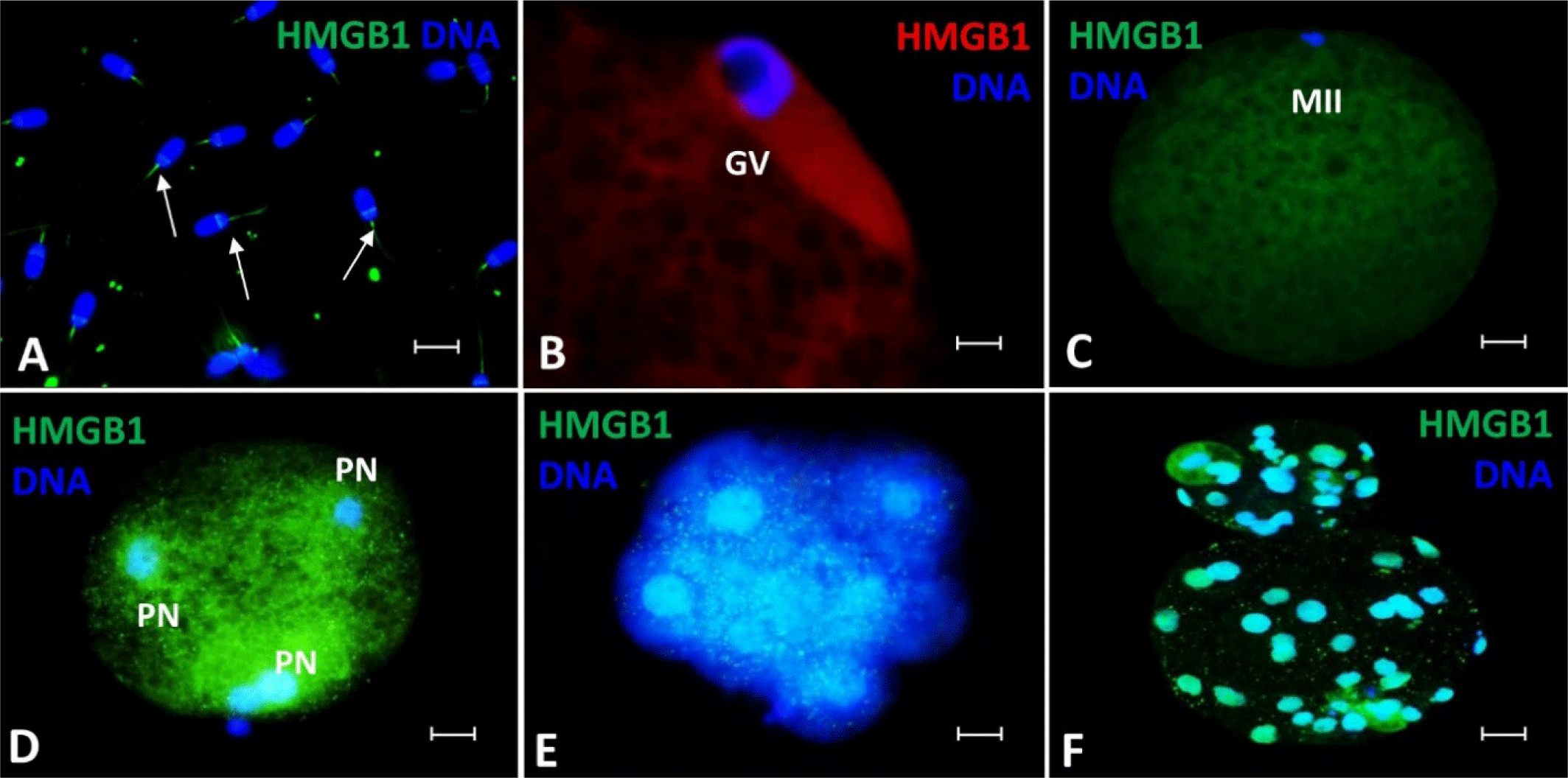
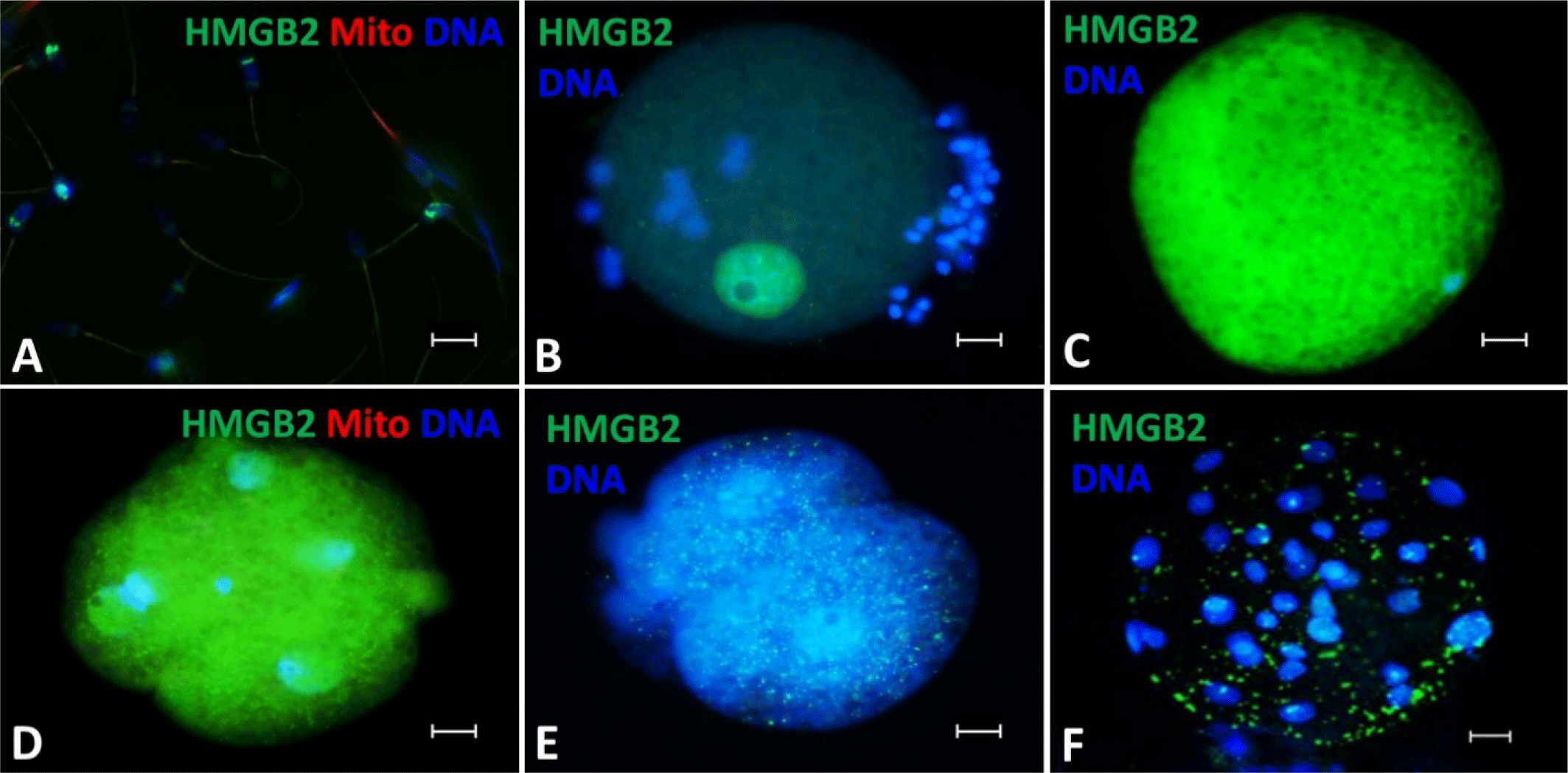
As shown in Fig. 2A, HMGB2 was detected in the post-acrosomal sheath and tail of spermatozoa. In the germinal vesicle breakdown (GVBD) stage, abundant HMGB2 was visualized in the nucleus (Fig. 2B). Similarly, it was strongly expressed in the cytoplasm after the maturation of the oocytes (Fig. 2C). After IVF, HMGB2 localization was detected in the pronucleus (Fig. 2D), the zygote nucleus (Fig. 2E), and the nuclei of pre-implantation embryonic cells (Fig. 2F). Similar to HMGB1 localization, HMGB2 showed strong expression in the pronuclei and cytoplasmic regions during the post-fertilization pronuclear formation stage (Fig. 2D). Cytoplasmic expression decreased somewhat (Fig. 2E), but HMGB2 localization was again clearly observed in the nuclei and cytoplasm of pre-implantation blastocysts (Fig. 2F).
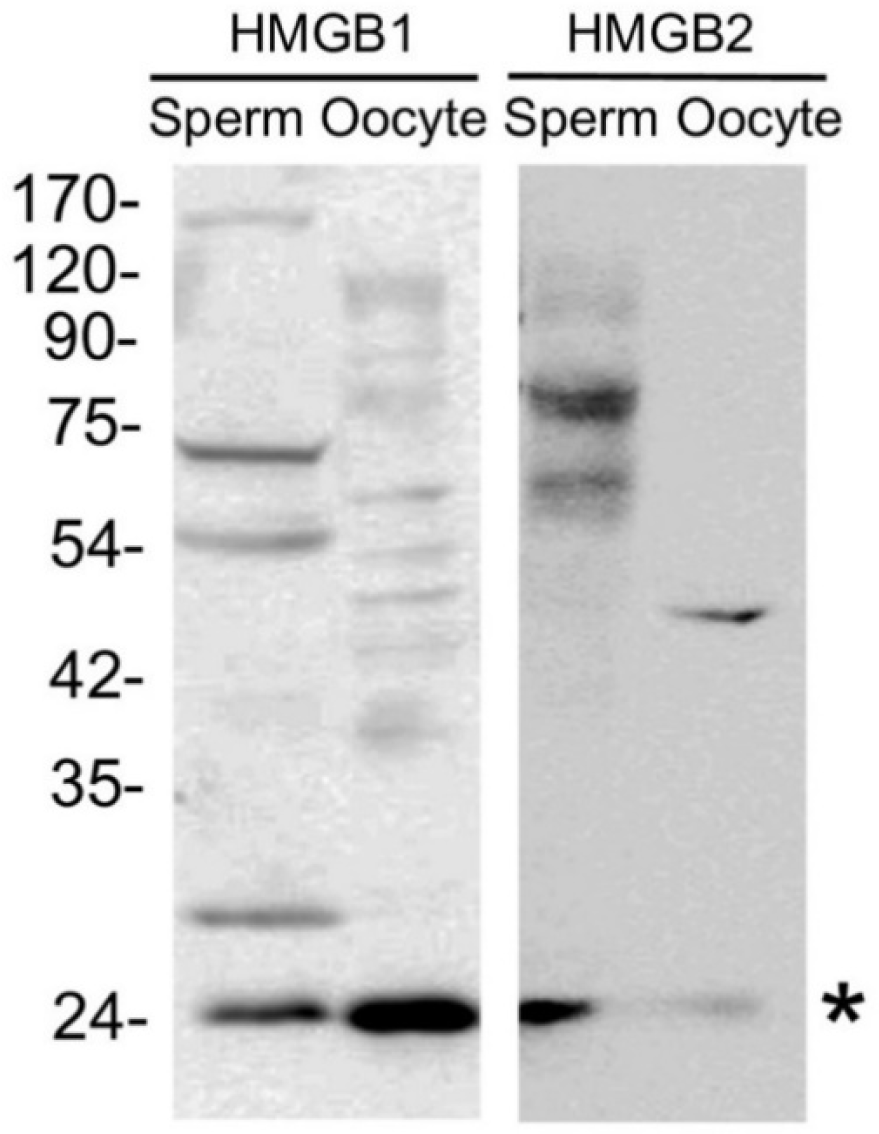
Western blot analysis was performed using antibodies against HMGB1 and HMGB2 on protein extracts from the spermatozoa and oocytes. HMGB1 and HMGB2 were detected at approximately 25 kDa in both spermatozoa and oocytes. At the upper part of the band, protein degradation was observed in the complex binding form of HMGB1 and HMGB2 (Fig. 3).
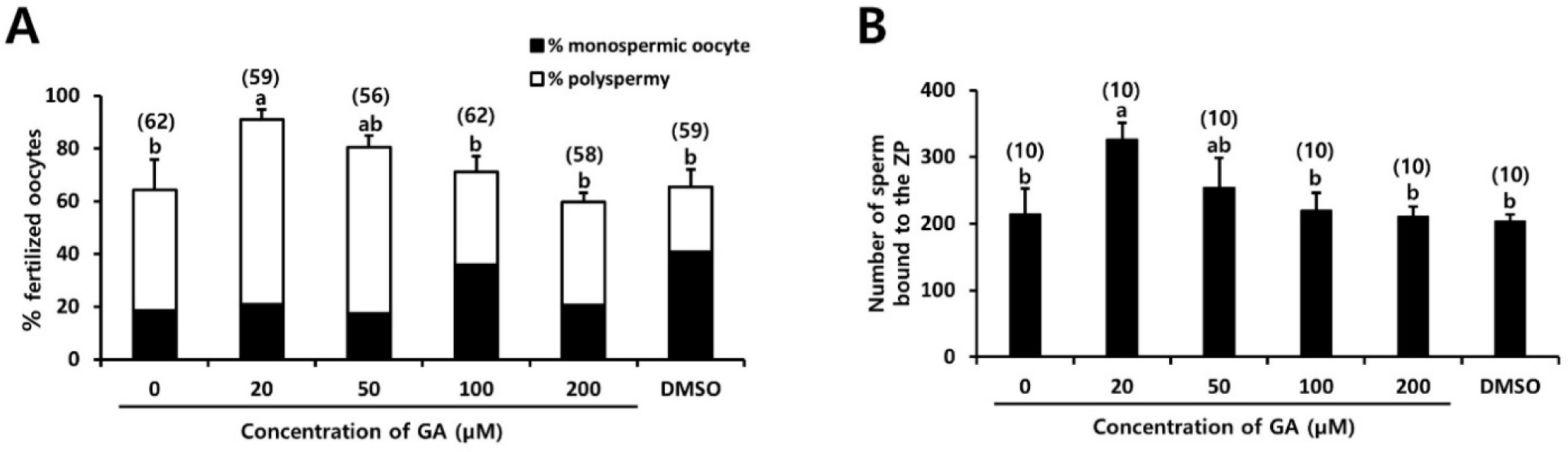
To determine the fertilization rates, the oocytes were subjected to insemination with spermatozoa in the presence of GA for 5 hr, followed by a 20 hr culture period (Fig. 4A). Additionally, one hr after IVF, other batches of oocytes were fixed and stained using DAPI to count the number of spermatozoa bound to the zona pellucida (ZP; Fig. 4B). In Fig. 5A, the total fertilization rate can be observed, which encompasses both monospermic and polyspermic oocytes. There was no significant difference in the rate of normal fertilization (monospermic oocytes; 18.6%–40.7%), while the polyspermy rate increased significantly in oocytes fertilized in the presence of 20 µM GA (70.3%, p<0.05) compared to oocytes fertilized with (50–200 µM GA) or without GA (24.8%–63.2%, Fig. 4A). Consequently, a higher rate of total fertilization was observed in oocytes fertilized with 20 µM GA (91.1%, p<0.05, Fig. 4A). After co-culturing the sperm and oocytes in medium with (20–200 µM GA) or without GA for 1 hr, the number of sperm attached to the ZP of the oocytes was counted. The results showed that a higher number of sperm bound to the oocytes in the medium containing 20 µM GA (326.5 vs. 203.3–253.8, p<0.05, Fig. 4B).
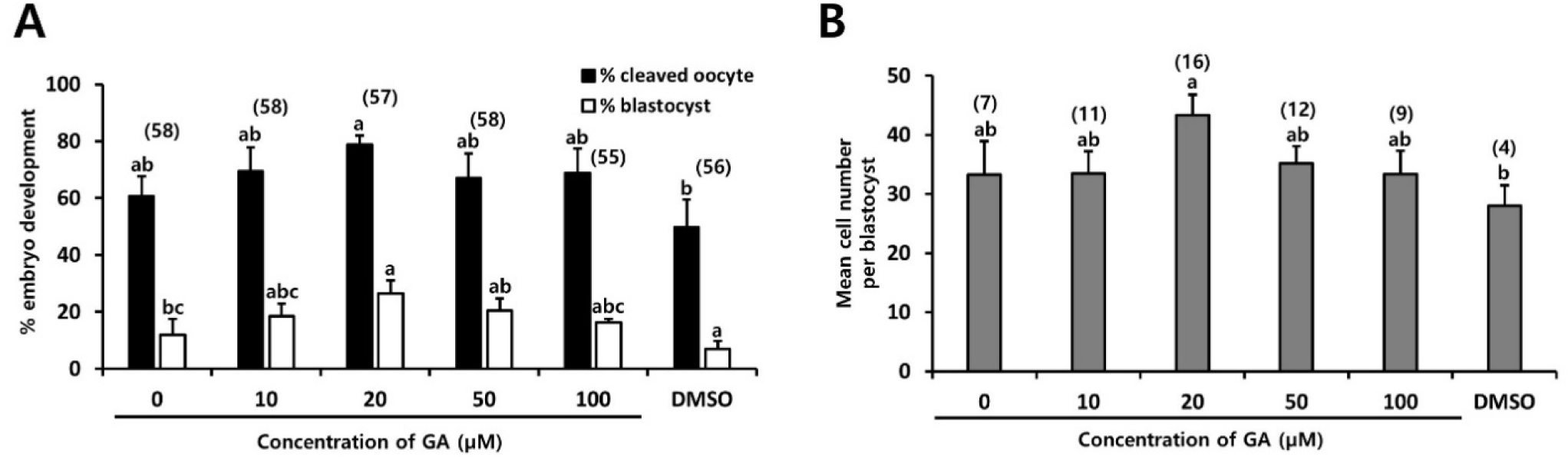
After IVF, the fertilized oocytes were subjected to culture in the PZM-3 medium in the absence or presence of various concentrations of GA (10–100 µM) for 48 and 144 hr (Fig. 5). The percentage of cleaved embryos was significantly higher in fertilized oocytes cultured in the presence of 20 µM GA (78.8%, p<0.05) than those in fertilized oocytes cultured with (10, 50 and 100 µM GA) or without GA (49.8%–69.5%, Fig. 5A). The blastocyst formation rate was also significantly higher in the fertilized oocytes cultured in PZM-3 medium containing 20 µM GA (26.5%, p<0.05) than in the other groups (7.0%–20.5%, Fig. 5A). A higher mean cell number was observed in the blastocyst in the presence of 20 µM GA (43.3 vs. 28–35.2, p<0.05, Fig. 5B). These results indicate that the addition of 20 μM of GA in the culture medium may have a significant influence on cleavage, blastocyst formation, and the average cell count of blastocysts. This suggests that GA has the potential to enhance the development of porcine embryos during IVC.
DISCUSSION
In this study, following IVF and IVC with the addition of GA, improvements were observed in certain parameters associated with oocyte and embryo development. The results showed the ability of GA to suppress HMGB1. It is known that HMGB1 is an evolutionarily conserved protein, widely distributed in the nuclei and cytoplasm of nearly all cell types [8]. HMGB1 consists of two contiguous DNA-binding domains, specifically the HMG A box (comprising 9–79 amino acids) and the B box (comprising 89–162 amino acids), along with a C-terminal tail (comprising 186–215 amino acids) that possesses a significantly negative charge. It has been shown that HMGB1 has an N-terminal region that plays a key role in cellular processes involving DNA regulation, repair, and gene transcription [8, 18, 19]. As previously mentioned, HMGB1 binds to chromatin and is predominantly found in the nucleus. It is capable of moving from the nucleus to the cytoplasm and to extracellular vesicles in response to high levels of reactive oxygen species (ROS) production [20]. The presence of higher concentrations of free oxygen radicals suggests that increased oxidative stress could reduce the rate of embryonic development [21]. ROS are produced through IVF culture media as a result of high oxygen concentration, metabolic activities of oocytes and embryos, and influence on both oocyte maturation and embryo development [22]. Furthermore, free radicals have the potential to damage various cellular components, including DNA, adenosine triphosphate synthesis, and the mitotic spindle [23]. The higher level of HMGB1 expression in endometrial epithelial cells can diminish the adhesive capacity of these cells, thereby impacting blastocyst implantation. It has been suggested that the amount of HMGB1 protein in the follicular fluid is linked to the growth of the follicle and the success of IVF [24] and that higher levels lead to repeated implantation failure [25]. Therefore, the increase in HMGB1 levels during embryo development necessitates the development of a defense mechanism against HMGB1.
GA is a natural compound commonly found in licorice roots [12]. It is a triterpene diol conjugate that forms a direct bond with HMGB1 [26] when GA binds two flat curved surfaces of HMG boxes that are created by hydrophobic and electrostatic interactions with key residues, while its sugar moieties are directed outward [27]. The expression level of HMGB1-TLR4-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) mRNA can be inhibited by GA, and molecular docking studies have revealed that GA has an antiepileptic effect to suppress the inflammatory conditions [28]. Furthermore, GA has demonstrated significant potential in the treatment of pregnancy-related complications, such as reducing endothelial cell permeability in preeclampsia and suppressing HMGB1–induced inflammation in hypoxic trophoblastic tissue, establishing itself as a valuable agent in maintaining maternal and fetal health [1]. In this study, HMGB1 and HMGB2 were detected in spermatozoa, oocytes, and embryonic nuclei by immunofluorescence (Figs. 1 and 2). Similarly, in an earlier study, HMGB1 and HMGB2 were detected in the mice testis using immunohistochemistry, with a complementary expression pattern. Thus, HMGB2 expression was high in spermatocytes and elongated spermatids, while HMGB1 expression was low in spermatocytes and absent in spermatids [29]. Also, the HMGB1 expression was high in zygotes and decreased in the 2-cell stage of embryos, and again increased gradually during the development to the morula and blastocyst stage in mice [30].
Polyspermy frequently occurs in IVF of pig oocytes, and these embryos face difficulties in developing to the blastocyst stage [31]. Our study found a significantly higher (p<0.05) rate of total fertilization (mono and polyspermic oocyte) in 20 μM of GA compared to the control following IVF. Similarly, in another study, the IVF rate was significantly increased (p<0.05) with aqueous licorice extract (Glycyrrhiza uralensis), with an optimal concentration of 0.02 mg/mL, using sperm from BALB/c mice [15]. Another study found that sperm from BALB/cA mice preincubated with 0.03 mg/mL licorice extract showed an excellent concentration of IVF rate [32]. Similarly, a significantly higher (p<0.001) fertilization rate was observed with 10% Glycyrrhiza glabra extract treatment when compared with the control group in mice during IVF. These results are important as they help identify the substances in licorice that influence the efficacy of IVF [33]. We found that there was a significant increase in the percentages of cleaved embryos and blastocyst formation in the presence of 20 µM GA, and the mean cell numbers per blastocyst were also significantly higher in embryos cultured with 20 µM GA compared with the control group (p<0.05). In a previous study, an optimal percentage of two-cell embryos was achieved with 0.02 mg/ml of licorice extract containing isoliquiritigenin and formononetin after IVF in mice [15]. Licorice root has approximately 500 components, and the main active ingredients are GA and several flavonoids. Earlier reports suggested that the addition of GA alone to the incubation medium had no significant effect on sperm fertilization ability, and that other components of licorice, either alone or combined with GA, may result in an increase in IVF rates without any damage to fertilized eggs in mice in vitro. This suggests that licorice extract maintains sperm motility in the medium but does not induce the acrosome reaction, thereby potentially improving fertilization rates by prolonging sperm penetration time into the oocyte [32]. In our experiment, we observed improved fertilization and blastocyst formation rates following a single treatment of GA in IVF or IVC medium (Fig. 5). This suggests that GA may modulate HMGB1, which is expressed during the early stages of embryo development [30]. In conclusion, the presence of HMGB1 and HMGB2 was confirmed in porcine germ cells, and it was found that adding GA, an HMGB inhibitor, to IVF or IVC media increased fertilization rates and preimplantation blastocyst formation rates. This suggests that GA could be utilized as a potential agent to enhance fertilization and embryonic development in assisted reproduction.