INTRODUCTION
Myxomatous mitral valve disease (MMVD) is the most prevalent cardiac disease in dogs, accounting for more than 70% of all canine heart disease [1]. According to the American College of Veterinary Internal Medicine (ACVIM) guidelines, MMVD is divided into four basic stages (A, B, C, and D) based on the risk of heart failure, cardiac remodeling, clinical signs, breed-adjusted radiographic vertebral heart score (VHS) and responsiveness to standard treatment [2]. Dogs with stage B disease have structural abnormalities of the heart but no clinical signs of heart failure. Stage B is divided into B1 and B2 by the following criteria related with heart enlargement: murmur intensity ≥ 3/6, echocardiographic left atrial-to-aortic (LA/Ao) ratio ≥ 1.6, and left ventricular end diastolic diameter normalized for body weight (LVIDDn) ≥ 1.7 [2].
As the disease progresses, chronic mitral valve regurgitation leads to volume overload, primarily characterized by left atrial enlargement (LAE) [3]. LAE is associated with a risk of congestive heart failure and poor prognosis [4, 5]. The LA/Ao ratio in the right parasternal short-axis view on echocardiography is commonly used to assess LAE in dogs [2, 3]. It has certain limitations, such as defining the aortic measurement in relation to the valve sinus, the exclusion of pulmonary veins from the LA measurement, and the requirement of consistent timing of LA measurement during the cardiac cycle [6]. Additionally, the LA/Ao ratio is affected by variations in aortic dimensions, and a higher level of interoperator measurement variability compared with the left atrial anteroposterior diameter (LAD) was reported [7–10].
In cats, horses, and humans, the LAD is obtained from the parasternal long-axis view on echocardiography and is commonly used to evaluate LA size [11–13]. However, the LAD has not been widely used in dogs, with few studies exploring its use. In one study, the left atrial anteroposterior diameter normalized to body weight (LADn) in dogs based on an allometric scale was established. Results showed that LADn increased as the severity of MMVD progressed [1].
N-Terminal pro-brain natriuretic peptide (NT-proBNP) is a cardiac biomarker of myocardial function and congestive heart failure [2]. It is produced by cardiac muscles and released in response to volume overload, hypertrophy, and hypoxia [3]. High NT-proBNP levels are related to heart volume and poor prognosis of patients: > 800 pmol/L indicates a high probability of heart disease, > 1,800 pmol/L suggests the presence of heart disease, and > 2,700 pmol/L indicates congestive heart failure [2, 3]. However, the concentration of NT-proBNP can be affected by concurrent diseases such as renal dysfunction, systemic and pulmonary hypertension, infectious disease, severe arrhythmia, sepsis, and pulmonary thromboembolism [3, 4].
The purpose of our study was to establish a correlation between LADn and NT-proBNP levels in dogs with MMVD and to assess the diagnostic potential of LADn for MMVD.
MATERIALS AND METHODS
This study included 35 client-owned dogs that visited Jeonbuk National University Animal Medical Center between February 2023 and September 2023. This study was approved by the Institutional Animal Care and Use Committee (approval number: NON2023-173).
Participants included 10 control dogs and 25 dogs with MMVD. The control group included mixed-breed dogs (n = 3), Cocker spaniels (n = 3), French bulldogs (n = 2), Pekinese dog (n = 1), and poodle dog (n = 1). The MMVD group included Maltese dogs (n = 8), poodles (n = 5), Cocker spaniels (n = 3), miniature Schnauzers (n = 2), mixed-breed dogs (n = 2), Chihuahuas (n = 2), Pomeranian (n = 1), miniature Pincher (n = 1), and Yorkshire terrier (n = 1). MMVD was diagnosed based on physical examination, thoracic radiography, and echocardiography. Dogs with MMVD were categorized into stages B1, B2, or C according to the ACVIM consensus guidelines [5].
Exclusion criteria included the presence of systemic hypertension (systolic blood pressure ≥ 160 mmHg consistently over multiple measurements and requiring antihypertensive treatment), clinically important arrhythmia (requiring anti-arrhythmic medication or pacemaker implantation), congenital cardiac disease, pulmonary hypertension (tricuspid regurgitation jet velocity > 3 m/sec), and azotemia (creatinine > 1.40 mg/dL).
2 mL of whole blood using serum separator tube was collected from the cephalic and jugular veins of all dogs. The serum samples were left to coagulate for 10 min and then centrifuged at 3,500 rpm (~1,800 × g) for 10 min. The centrifuged serum was separated into Eppendorf tubes and stored in an ultracold (−80°C) freezer until analysis. NT-proBNP concentration was measured using a BIONOTE V200 fluorescent immunoassay system (BIONOTE, Hwaseong, Korea). The NT-proBNP reference level of this equipment ranged f rom 500 to 10,000 pmol/L.
Thoracic radiographs were taken to obtain ventrodorsal and right-lateral views. The VHS and vertebral left atrial size (VLAS) were measured to evaluate the cardiac size on the right lateral view, as described in previous studies [6, 7]. We classified the criteria for cardiac enlargement based on the general breed standards outlined in the ACVIM guideline (VHS ≥ 11.5). When the results for cardiac enlargement differed between VHS and echocardiographic indices (LA/Ao, LVIDDn), we classified the MMVD stage based on the echocardiographic findings.
The pulmonary vessel size and state parenchyma were also evaluated to estimate the possibility of congestive heart failure.
All echocardiographic studies were performed using a Philips EPIC 7C echocardiography machine (Philips, Netherlands) with 3–8 and 4–12 MHz phased transducers.
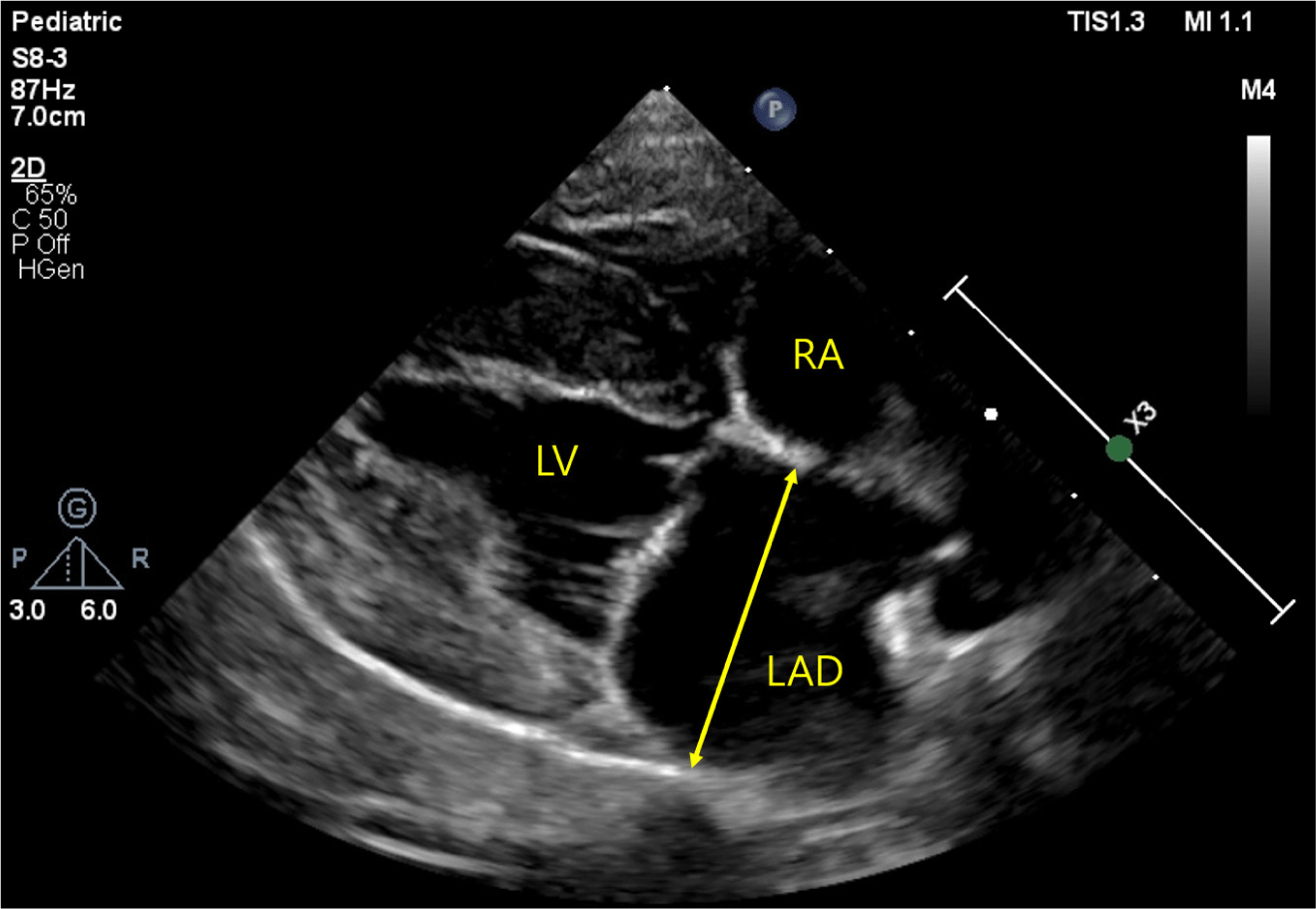
In all dogs, the LAD was measured at end-systole, utilizing the right parasternal long-axis view (Fig. 1) [9]. The measurement was conducted by evaluating the widest distance parallel to the mitral annulus from the inner wall of the middle interatrial septum to the inner wall of the posterior free wall [1]. LADn was calculated as LAD (mm) / body weight (kg)0.324 [1]. The LA/Ao ratio, LVIDDn, and E peak velocity were measured to estimate cardiac remodeling and function, as described in previous studies [8].
Statistical analyses were conducted using commercially available statistical software (IBM SPSS Statistics 29.0, IBM, Armonk, NY, USA). The Shapiro-Wilk and Kolmogorov-Smirnov methods were used to check the normality of the data. To compare values among the four groups, analysis of variance (ANOVA; age, weight, VHS, VLAS, LA/Ao ratio, LVIDDn, E peak velocity, LAD, and LADn) and the Kruskal-Wallis test (NT-proBNP) were used, and post-hoc tests with Bonferroni correction were performed. Additionally, Spearman’s correlation coefficients (r) were used to validate the association between the LADn and other variables, including NT-proBNP. Statistical significance was set at p<0.05.
RESULTS
A total of 35 dogs were enrolled in this study: 10 control dogs and 25 dogs with MMVD. The MMVD group was categorized according to the ACVIM stage criteria, with 10 dogs in stage B1, 10 dogs in stage B2, and 5 dogs in stage C. The demographic, radiographic, and echocardiographic data for all groups are summarized in Table 1. The age of dogs with MMVD was significantly higher (11.36 years, range: 4–17 years) than that of control dogs (5.5 years; range: 1–9 years; p<0.001). Furthermore, dogs with MMVD exhibited a reduced body weight (5.06 kg; range: 1.9–10 kg) when compared to control dogs (11.4 kg; range: 4.97–25 kg; p=0.006).
ACVIM, American College of Veterinary Internal Medicine; VHS, vertebral heart size; VLAS, vertebral left atrial size; LA/Ao ratio, left atrial-to-aortic ratio; LVIDDn, left ventricular end-diastolic diameter normalized to body weight; LAD, left atrial anteroposterior diameter; LADn, left atrial anteroposterior diameter normalized to body weight.
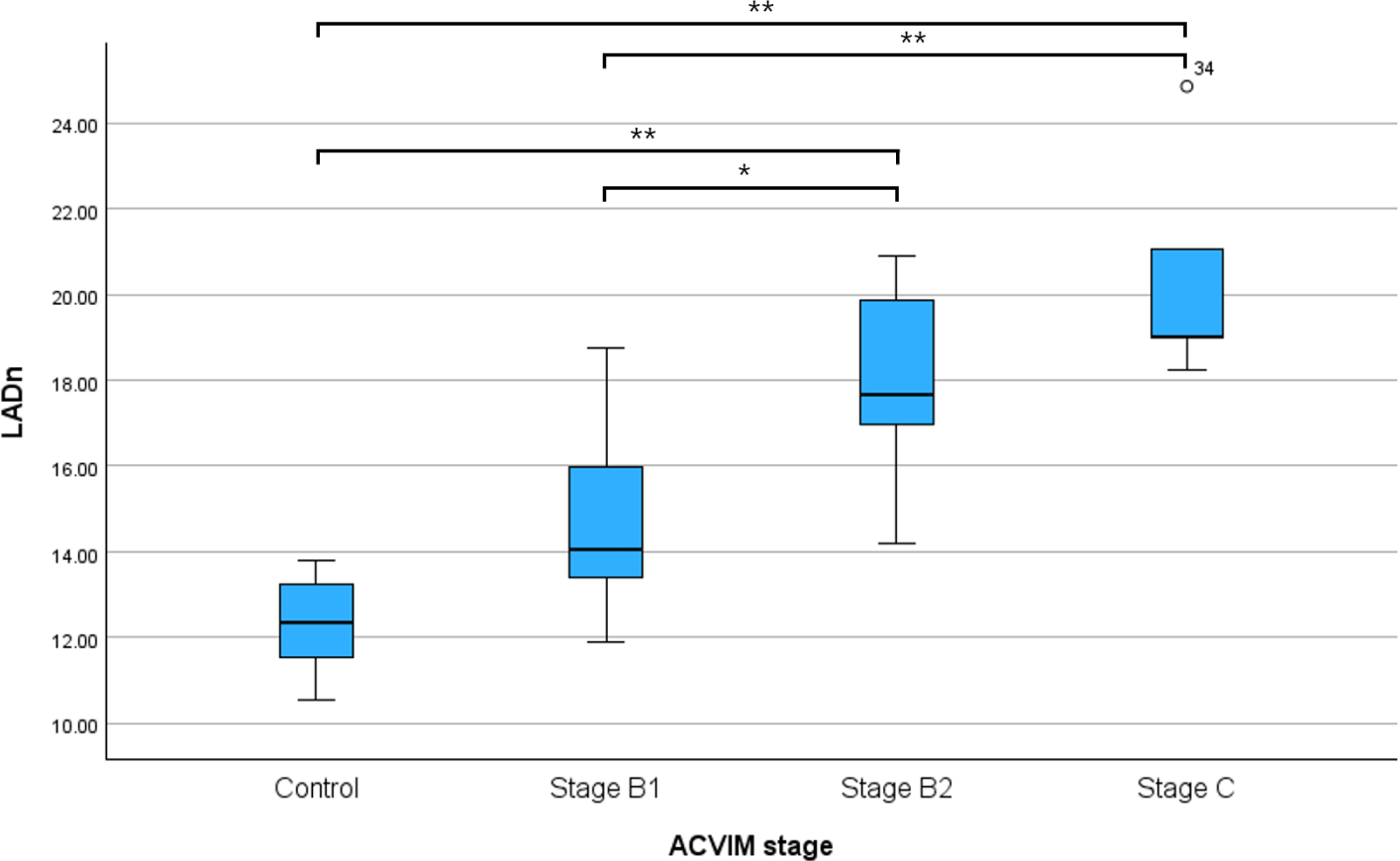
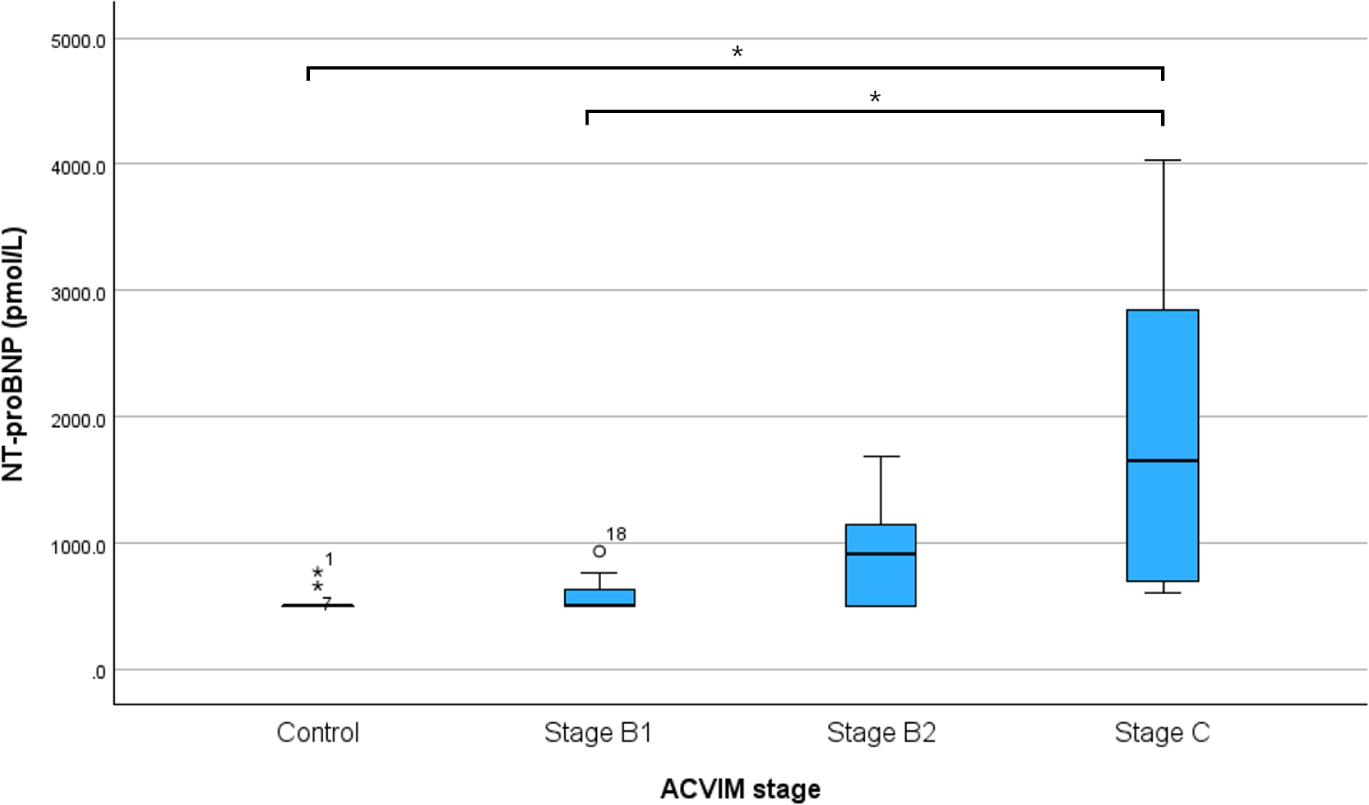
The LADn of Dogs with MMVD was higher than that of control dogs, and the difference in LADn according to MMVD stage is shown in Table 1 and Fig. 2. LADn was significantly different between the control and B2 groups (p<0.001), control and C groups (p<0.001), B1 and B2 groups (p=0.004), and B1 and C groups (p<0.001). NT-proBNP were significantly different between control and C groups (p=0.009) and B1 and C groups (p=0.025; Fig. 3).
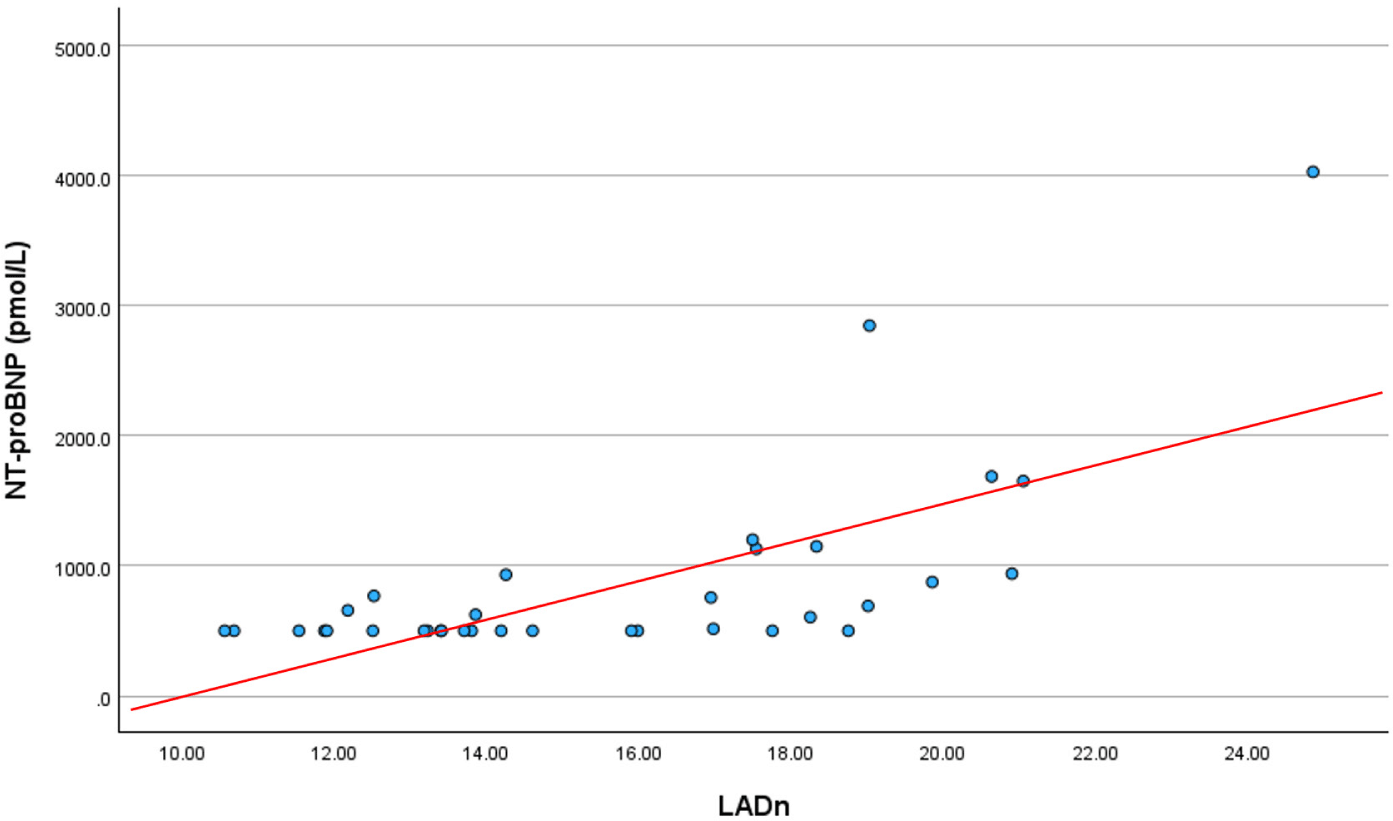
The correlations between LADn and the other values are summarized in Table 2. LADn positively correlated with age, VHS, VLAS, LA/Ao ratio, LVIDDn, E peak velocity, and NT-proBNP. Conversely, LADn negatively correlated with weight (p=–0.462). NT-proBNP positively correlated with VHS, VLAS, LA/Ao ratio, LVIDDn, E-peak, LAD, and LADn (p<0.05). We observed a strong positive correlation between the LADn and NT-proBNP levels (Fig. 4), with the correlation coefficient rs= 0.69.
LADn, left atrial anteroposterior diameter normalized to body weight; LA/Ao ratio, left atrial-to-aortic ratio; LVIDDn, left ventricular end-diastolic diameter normalized for body weight; LAD, left atrial anteroposterior diameter; VHS, vertebral heart size; NT-proBNP, N-terminal fragment of prohormone B-type natriuretic peptide; VLAS, vertebral left atrial size.
DISCUSSION
This study focused on the differences in LADn according to the MMVD stage and the correlation between LADn and NT-proBNP levels. In a recent study, an increase in LADn was observed with the progression of ACVIM stages. This study also reported similar findings [14]. There were significant differences in the LADn for each group. In recent studies, NT-proBNP levels increased as ACVIM stage increased, and similar results were observed in this study [15, 16]. There was a strong positive correlation between the LADn and NT-proBNP levels. Moreover, LADn was significantly correlated with VLAS, LA/Ao ratio, and LVIDDn. These results suggest that LADn can be an indicator of ACVIM stage.
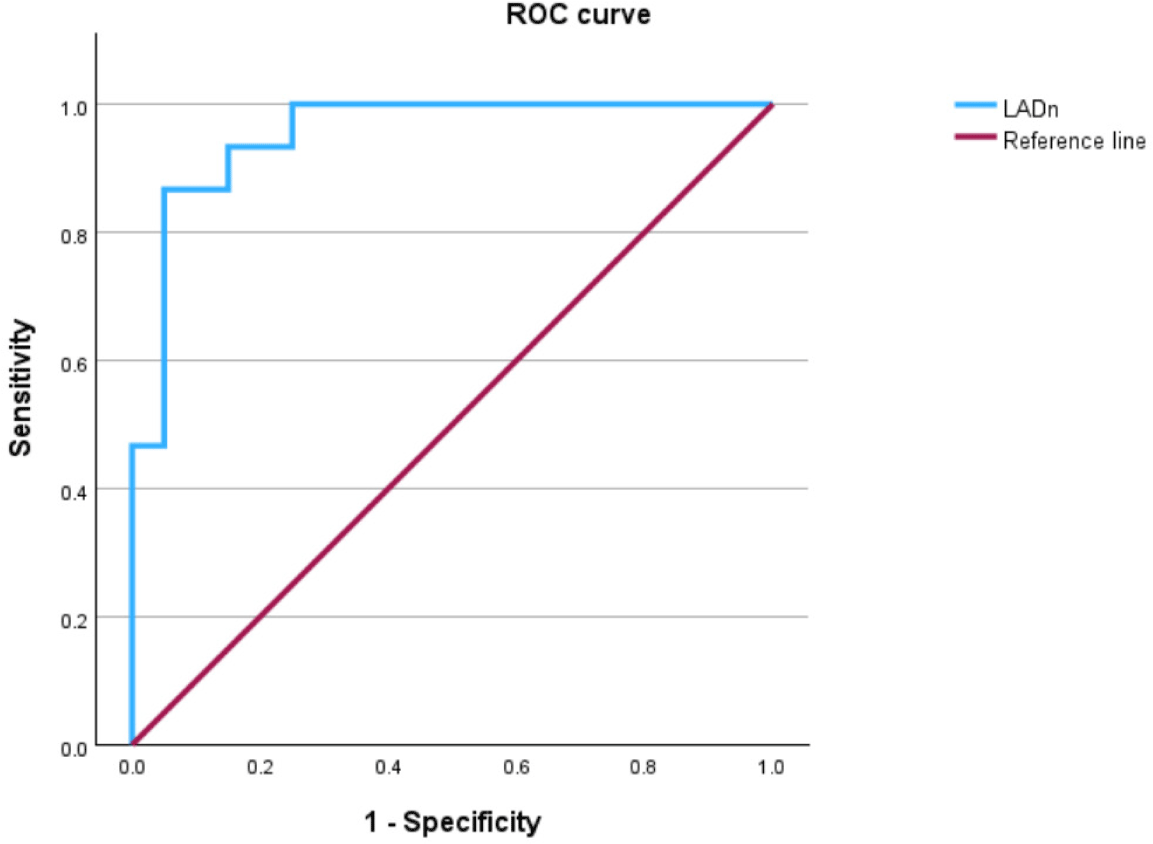
The mean LADn value in the control group was 12.22 ± 1.1, and it showed a significant increase when comparing stage B2 with stage C (p<0.05) as well as among stages B1, B2, and C (p<0.05). Receiver operating characteristic (ROC) curve analysis demonstrated that an elevated LADn value can be a highly accurate predictor for differentiating between dogs in the non-cardiac remodeling groups (control and B1) and cardiac remodeling groups (B2 and C; AUC, 0.953 [95% confidence interval, CI], 0.888–1.018, p<0.001; Fig. 5). An LADn cutoff value of 16.96 yielded the highest combined sensitivity (87%) and specificity (95%). An LADn cut-off value of 17 could be used to identify dogs with ACVIM stage B2.
The LAD method has shown lower measurement variability than the LA/Ao method in previous studies [10, 14]. In one study, the interobserver measurement variability of the LAD was significantly lower (4.2%) than that of the LA/Ao ratio (10.9%) [10]. This may be due to the difficulty in achieving a consistent timing of aortic valve closure, a factor that influences the measurement of the LA/Ao ratio [17]. LAD measurement is affected by pulmonary veins, a factor that can affect LA/Ao ratio measurement. In one study, it was found that the LAD for estimating left atrial volume exhibited a strong correlation with the left atrial volume determined through real-time three-dimensional echocardiography [10]. The left atrium is a three-dimensional structure, and its enlargement may exhibit an asymmetrical pattern. The LADn can serve as a complementary tool to evaluate LA enlargement in dogs.
NT-proBNP is released in response to myocardial tissue compression and stress. It is commonly used for the diagnosis of heart disease and the assessment of the prognosis of heart failure. In this study, we found a strong correlation between NT-proBNP and LADn, suggesting that LADn may be a valuable indicator for evaluating left atrial size. In the case of NT-proBNP, there were no significant differences among the control, B1, and B2 groups; only the control, B1, and C groups showed significant differences. In addition, there was a substantial variation in level of NT-proBNP within the C group, which suggests that caution is needed when diagnosing or staging MMVD using only NT-proBNP.
This study had some limitations. Firstly, the number of dogs in each group was small. Further studies with larger and more diverse samples are required to obtain reliable data. Second, the effects of cardiac medications were not considered in groups B2, and C. Cardiac medications can influence myocardial stress and heart size. Third, the breed-specificity of the LADn was not analyzed. Although a prior study suggested that breed had no significant effect on LADn, further studies are needed to determine the need for breed-specific reference values [14]. Fourth, age was not taken into account. MMVD is a type of age-related degenerative disease commonly seen in dogs. Therefore, age could also be a factor influencing the results of this study. Future studies should consider age as well.
Finally, body weight was not considered in this study. In heart disease, body weight is one factor thought to be associated with prognosis. Therefore, it appears this factor should also be considered in futher study [18].
This study suggests that LADn may serve as a valuable diagnostic marker of MMVD in dogs. The strong positive correlation between LADn and NT-proBNP levels demonstrates the usefulness of LADn as a clinical index for evaluating cardiac function and disease progression in dogs with MMVD.