INTRODUCTION
Human dermal fibroblasts (HDFs) are essential for maintaining skin architecture through the production of extracellular matrix proteins and growth factors that support tissue integrity and repair [1]. However, under inflammatory or immune-mediated stress, such as that induced by activated T cells, fibroblasts undergo apoptosis and exhibit reduced motility, which impairs wound healing and accelerates skin aging [2, 3]. These processes are particularly relevant in chronic inflammatory conditions and aged skin, where fibroblast dysfunction contributes to impaired regeneration and structural degeneration of the dermis [2]. Therefore, identifying strategies to protect fibroblasts from immune-mediated damage has important implications for skin aging and chronic wound management.
In recent years, adipose-derived stem cells (ADSCs) have emerged as promising candidates for skin regeneration due to their capacity to secrete a broad repertoire of paracrine factors, including cytokines, chemokines, growth factors, and extracellular vesicles such as exosomes [4]. ADSC-derived exosomes are nano-sized (30–150 nm) lipid vesicles that contain proteins, lipids, and nucleic acids reflective of their cell of origin, and have been shown to mediate intercellular communication in tissue repair and immune modulation [5]. Among their cargo, hepatocyte growth factor (HGF) is a well-known pleiotropic cytokine that promotes cell survival, proliferation, and motility through activation of its cognate receptor, c-Met [6].
Although the anti-apoptotic effects of ADSCs and their secretome have been studied in nutrient-deprived fibroblasts [7], little is known about the role of exosomal HGF in protecting fibroblasts from immune-mediated stress. Furthermore, whether exosomal HGF contributes not only to fibroblast survival but also to enhanced migration under inflammatory conditions remains largely unexplored. Given that T cell-derived cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) can promote fibroblast apoptosis [8], and that fibroblast migration is crucial for effective dermal remodeling, understanding how ADSC-derived exosomes regulate these processes may provide important therapeutic insight. Unlike previous studies limited to serum-deprivation models [7], our approach focuses on immune-mediated fibroblast injury, thus more closely reflecting chronic inflammatory skin conditions.
In this study, we aimed to investigate the dual role of ADSC-derived exosomal HGF in regulating apoptosis and migration of fibroblasts exposed to T cell-mediated inflammatory stimuli. We employed a Jurkat T cell co-culture model, which recapitulates T cell-driven dermal inflammation in vitro, to mimic immune-induced fibroblast stress. We then examined the protective and motogenic effects of ADSC-derived exosomes. We further explored the underlying signaling mechanisms with a focus on the AKT and FAK pathways, which are known to mediate cell survival and motility, respectively. Our findings highlight a novel role of exosomal HGF as a central mediator of fibroblast viability and migration, with implications for the development of exosome-based regenerative therapies targeting inflamed or damaged skin.
MATERIALS AND METHODS
HDFs (PromoCell, Heidelberg, Germany) and ADSCs (PromoCell) were cultured following the supplier’s instructions. HDFs were maintained in Fibroblast Growth Medium 2 (PromoCell), and ADSCs were cultured in Mesenchymal Stem Cell Growth Medium 2 (PromoCell) at 37℃ in a humidified incubator with 5% CO₂. Jurkat T cells (ATCC TIB-152), a human T lymphocyte cell line, were cultured in RPMI-1640 medium (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. HDFs and ADSCs used in this study were from passages 3–5 and tested negative for mycoplasma contamination. Jurkat cells were routinely monitored for viability and maintained below 1 × 10⁶ cells/mL prior to use.
To evaluate T cell-mediated paracrine effects on HDFs, a transwell co-culture system was employed. HDFs were seeded in the lower chambers of 24-well plates at a density of 5 × 10⁴ cells/well and allowed to attach overnight. Jurkat cells (5 × 10⁴ cells/insert) were added to the upper chambers of 0.4 μm pore-size transwell inserts (Corning, Corning, NY, USA). T cell activation was induced by supplementing the medium in both upper and lower chambers with anti-CD3/CD28 antibodies (1 μg/mL each; BioLegend, San Diego, CA, USA). After 24 hr of co-culture, culture supernatants were collected for cytokine analysis, and HDFs were processed for apoptosis or downstream signaling assays. For experimental groups receiving ADSC-derived exosomes, exosomes were added to the lower chamber at indicated concentrations 1 hr prior to Jurkat cell addition. All experiments were performed in triplicate.
Apoptosis was assessed using the APOPercentage™ apoptosis assay kit (Biocolor, Carrickfergus, UK), following the manufacturer’s protocol. The assay specifically stains cells undergoing the membrane ‘flip-flop’ event, indicative of apoptotic but not necrotic death. Bright-field images of the pink-stained apoptotic cells were acquired, and apoptosis was quantified by calculating the number of stained pixels using Adobe Photoshop. The degree of apoptosis was expressed as total red-pixel count per field.
After 24 hr of HDF-Jurkat co-culture, the Transwell inserts containing Jurkat cells were removed, and supernatants from the lower chambers were collected. Concentrations of interleukin (IL)-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α were quantified using human ELISA kits (R&D Systems, Minneapolis, MN, USA). Conditioned media from HDFs treated with ADSC-derived exosomes (no Jurkat present) were harvested at 24, 48, and 72 hr. Levels of HGF, insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β) were measured with the corresponding ELISA kits from the same vendor. All samples were assayed in duplicate and normalized to total cell number; results are reported as pg/mL.
HDFs were lysed using RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations were determined using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Darmstadt, Germany). Membranes were blocked with 5% skim milk in TBST for 1 hr at room temperature and incubated overnight at 4℃ with primary antibodies against phospho-AKT, total AKT, phospho-FAK (Tyr397), total FAK, MMP-2, phospho-ERK1/2, total ERK1/2, phospho-p38, total p38, and β-actin (Cell Signaling Technology, Danvers, MA, USA). HRP-conjugated secondary antibodies (Cell Signaling Technology) were used at 1:5,000 dilution. Signals were developed using Clarity Western ECL Substrate (Bio-Rad Laboratories, Hercules, CA, USA) and visualized with a ChemiDoc™ Imaging System (Bio-Rad Laboratories).
Exosomes were isolated from ADSC-conditioned media using the exoEasy Maxi Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. Briefly, conditioned medium was cleared by centrifugation at 500 × g for 10 min and 3,000 × g for 15 min to remove cells and debris. The supernatant was mixed with binding buffer and passed through an exoEasy membrane affinity spin column. After washing, exosomes were eluted with elution buffer and stored at –80℃ until use. Exosomal marker expression (CD9, CD63, TSG-101) and the absence of the cellular protein calnexin were confirmed by Western blot analysis using validated antibodies (e.g., CD9, CD63, TSG-101: Abcam, Calnexin: Cell Signaling Technology).
To test HGF’s role in exosomal function, ADSCs were transfected with HGF siRNA (Santa Cruz Biotechnology, Dallas, TX, USA) at 25 nM using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA). Transfection was performed in Opti MEM (Gibco), and cells were returned to standard culture medium after 6 hr. After 48 hr, supernatants were collected for exosome isolation as described above.
All experiments were performed in technical triplicate using the same lot of cells. Data are expressed as mean ± S.D. Statistical differences were analyzed using one-way ANOVA followed by Tukey’s post hoc test (GraphPad Prism 8.0, GraphPad Software, San Diego, CA, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
To mimic immune-mediated skin inflammation, HDFs were co-cultured with Jurkat cells using a transwell system, in the presence or absence of anti-CD3/CD28 antibodies. Anti-CD3/CD28 was employed to achieve polyclonal activation of Jurkat T cells via co-engagement of the T-cell receptor (CD3) and the CD28 co-stimulatory pathway, thereby eliciting robust cytokine secretion and effector signals that model T-cell–mediated inflammatory stress on fibroblasts; it does not directly act on HDFs, which do not express functional CD3 or CD28. After 24 hr of incubation, the inserts containing Jurkat cells were carefully removed so that all subsequent analyses reflected the response of the HDFs in the lower chamber. Apoptosis was evaluated after 24 hr using the APOPercentage™ assay. HDFs cultured alone exhibited minimal apoptosis, regardless of CD3/CD28 stimulation, suggesting that CD3/CD28 alone was insufficient to induce apoptosis in the absence of T cells. In contrast, co-culture with Jurkat cells significantly increased apoptotic staining in HDFs, and this effect was further augmented when Jurkat cells were activated with anti-CD3/CD28 antibodies (Fig. 1A) [9].
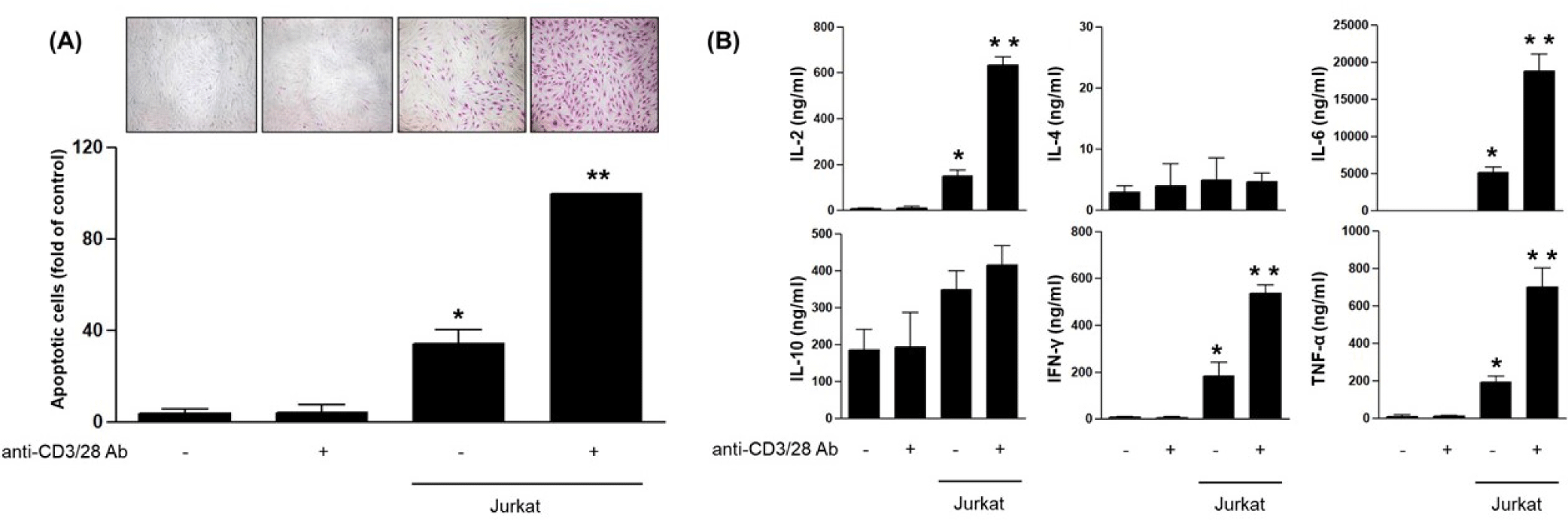
To determine whether these conditions also promoted inflammatory signaling, cytokine levels were measured in the culture supernatants after 24 hr. Co-culture with Jurkat cells led to a marked elevation of IL-2, IL-6, IFN-γ, and TNF-α concentrations. In contrast, IL-4 and IL-10 levels remained unchanged across all groups. These findings indicate that soluble pro-inflammatory cytokines released by activated T cells contribute to fibroblast apoptosis and inflammatory stress (Fig. 1B) [10].
To assess whether exosomes derived from ADSCs can mitigate T cell-induced fibroblast damage, ADSC-derived exosomes were isolated and characterized by Western blot. Strong expression of canonical exosome markers CD9, CD63, and TSG-101 confirmed successful isolation, while the endoplasmic reticulum protein calnexin was undetectable, confirming the absence of cellular contamination (Fig. 2A) [11]. Exosomes were then applied to the lower chamber of the HDF-Jurkat co-culture system. APOPercentage™ staining revealed that exosome treatment significantly reduced apoptosis in HDFs in a dose-dependent manner (Fig. 2B). In parallel, the motility of HDFs was evaluated using a transwell migration assay. Exosome treatment increased the number of migrated HDFs in a dose-dependent fashion, indicating that ADSC-derived exosomes also promote fibroblast migration under inflammatory conditions (Fig. 2C) [12].
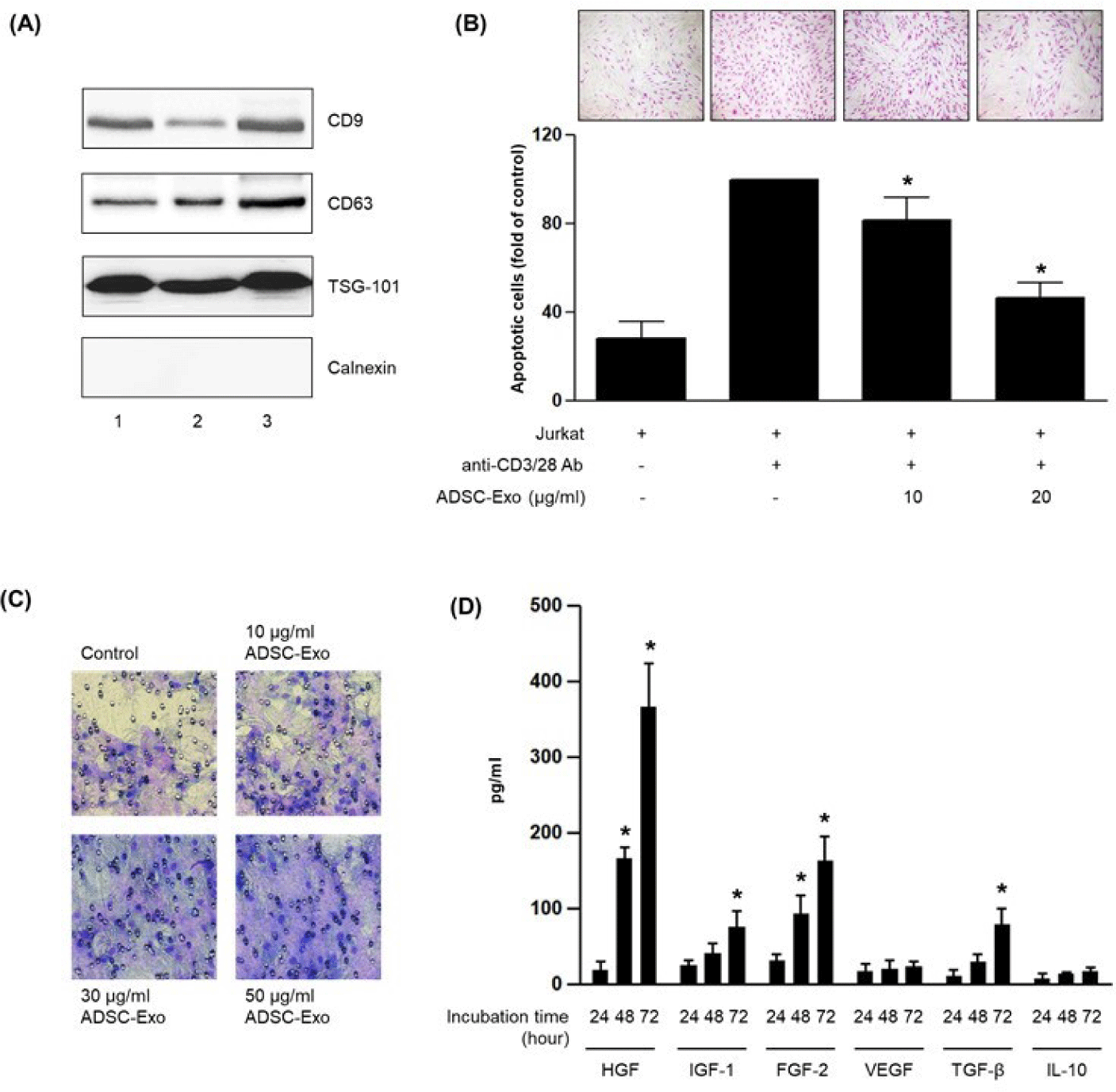
To further explore the functional consequences of exosome treatment on fibroblasts, we examined whether exosomes stimulate the secretion of regenerative growth factors. HDFs were cultured with ADSC-derived exosomes, and supernatants were collected at 24, 48, and 72 hr for analysis. ELISA revealed that levels of HGF, IGF-1, FGF-2, and TGF-β increased in a time-dependent manner. In contrast, VEGF and IL-10 levels showed no significant change during the same period (Fig. 2D) [13]. These results suggest that ADSC-derived exosomes stimulate fibroblasts to secrete specific growth factors associated with regeneration and repair.
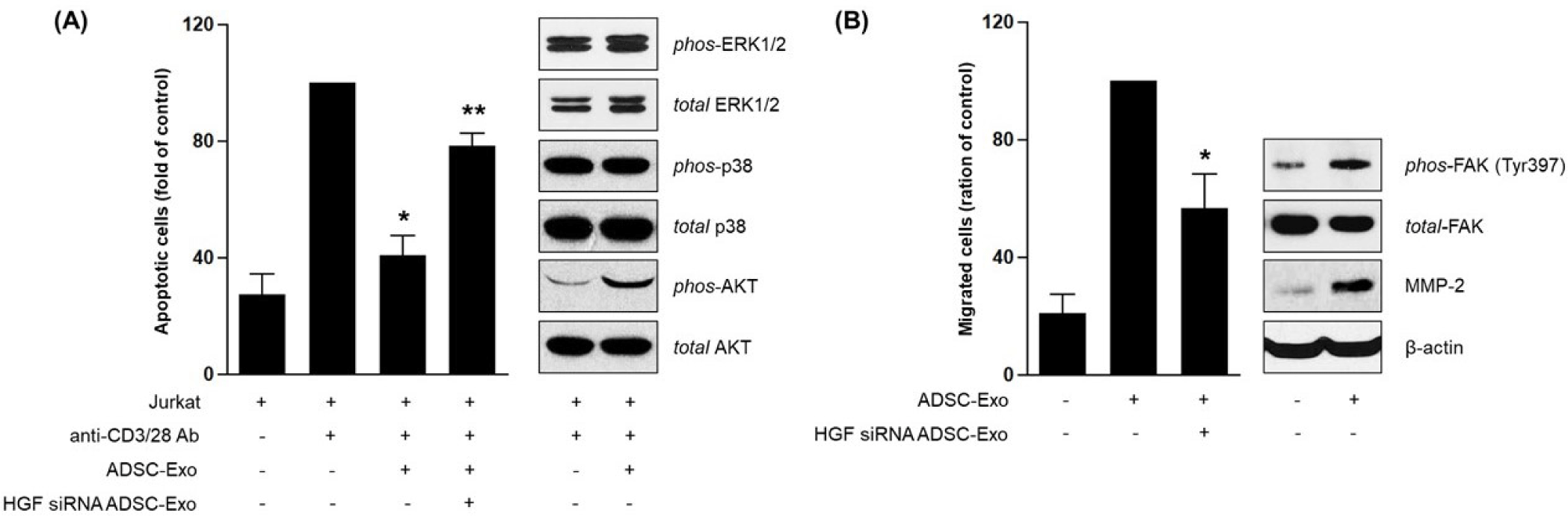
To determine whether the effects of ADSC-derived exosomes were mediated by HGF, siRNA was used to knock down HGF expression in ADSCs. Exosome treatment significantly reduced apoptosis in HDFs co-cultured with Jurkat cells, as expected. However, this protective effect was largely abolished in HGF-silenced cells, indicating that HGF is a critical mediator of the anti-apoptotic function (Fig. 3A) [14]. To investigate the associated signaling pathways, Western blot analysis was performed. Exosome-treated HDFs exhibited increased phosphorylation of AKT, but not ERK or p38, suggesting selective activation of survival signaling [15]. In migration assays, HGF knockdown also impaired the ability of exosomes to promote HDF migration. Further Western blotting revealed that exosome treatment enhanced phosphorylation of focal adhesion kinase (FAK) at Tyr397 and increased expression of MMP-2. Both signals were attenuated by HGF silencing, suggesting that exosomal HGF mediates fibroblast migration through the FAK-MMP axis (Fig. 3B) [16].
DISCUSSION
HDFs are central to the maintenance and repair of skin architecture, but their function can be severely compromised under inflammatory stress, leading to increased apoptosis and impaired migration. In this study, we established an in vitro model of immune-mediated fibroblast dysfunction using a transwell-based co-culture with Jurkat cells and anti-CD3/CD28 stimulation. This model induced significant apoptosis and a surge in pro-inflammatory cytokines (IL-2, IL-6, IFN-γ, TNF-α) in the culture supernatant, consistent with the known effects of T cell-derived signals on skin fibroblast pathophysiology [17]. Notably, HDFs alone did not exhibit cytokine induction or apoptosis under anti-CD3/CD28 treatment, supporting the paracrine nature of T cell-driven damage. This system offers a translationally relevant platform to study immune-induced dermal injury and potential regenerative interventions.
To counteract this inflammatory damage, we explored the therapeutic potential of exosomes derived from ADSCs, which are known for their immunomodulatory and trophic capabilities [18]. ADSC-derived exosomes were isolated using the exoEasy Maxi Kit, a column-based method that ensures high purity and reproducibility. The identity and quality of the exosomes were validated by Western blotting, demonstrating strong expression of CD9, CD63, and TSG-101, with the absence of calnexin indicating minimal contamination from cellular debris [19]. These findings confirm the integrity of the exosome preparations used in downstream experiments.
Functionally, ADSC-derived exosomes exhibited a dual regenerative effect on HDFs under T cell-induced stress. First, they significantly attenuated apoptosis in a dose-dependent manner, as shown by quantitative analysis of APOPercentage™ staining. Second, they enhanced HDF migration in transwell assays, suggesting their contribution to tissue repair mechanisms beyond cytoprotection. These effects were accompanied by a time-dependent increase in secretion of key growth factors (HGF, IGF-1, FGF-2, TGF-β) by fibroblasts in response to exosome treatment, while VEGF and IL-10 levels remained unchanged. This shift in secretome composition implies that exosomes not only act directly on fibroblast survival and motility but also reprogram their paracrine output to support dermal remodeling [20, 21].
A pivotal discovery in this study is the role of HGF carried by ADSC-derived exosomes. Using siRNA-mediated knockdown of HGF in ADSCs prior to exosome isolation, we demonstrated that the absence of exosomal HGF abrogated both the anti-apoptotic and pro-migratory effects on HDFs. Mechanistically, HGF-containing exosomes selectively activated AKT phosphorylation without affecting ERK or p38, highlighting the specificity of downstream survival signaling [22]. In parallel, exosomal HGF induced phosphorylation of FAK (Tyr397) and upregulation of MMP-2, supporting enhanced migratory capacity. Together, these data identify exosomal HGF as a principal mediator of the cytoprotective and pro-migratory effects, while cooperative contributions from additional exosomal factors cannot be excluded under inflammatory conditions. These observations establish a clear linkage between exosomal HGF and two critical fibroblast functions—survival and migration—through activation of the AKT and FAK pathways, respectively [23].
In summary, our findings provide compelling evidence that ADSC-derived exosomal HGF is a central mediator in fibroblast protection and motogenesis under immune-mediated stress. Exosomal HGF was necessary for full activity, acting as the dominant effector within a multifactorial exosomal milieu. This dual mechanism of action has important implications for cell-free regenerative therapies targeting inflammatory skin disorders and age-related dermal degeneration. Future studies should explore the therapeutic efficacy of HGF-enriched exosomes in in vivo models and investigate strategies for exosome engineering to enhance targeted delivery and potency [24].